Photomediated Transformation of Salannin, a Tetranortriterpenoid from Azadirachta indica A. Juss
Abstract
:Introduction
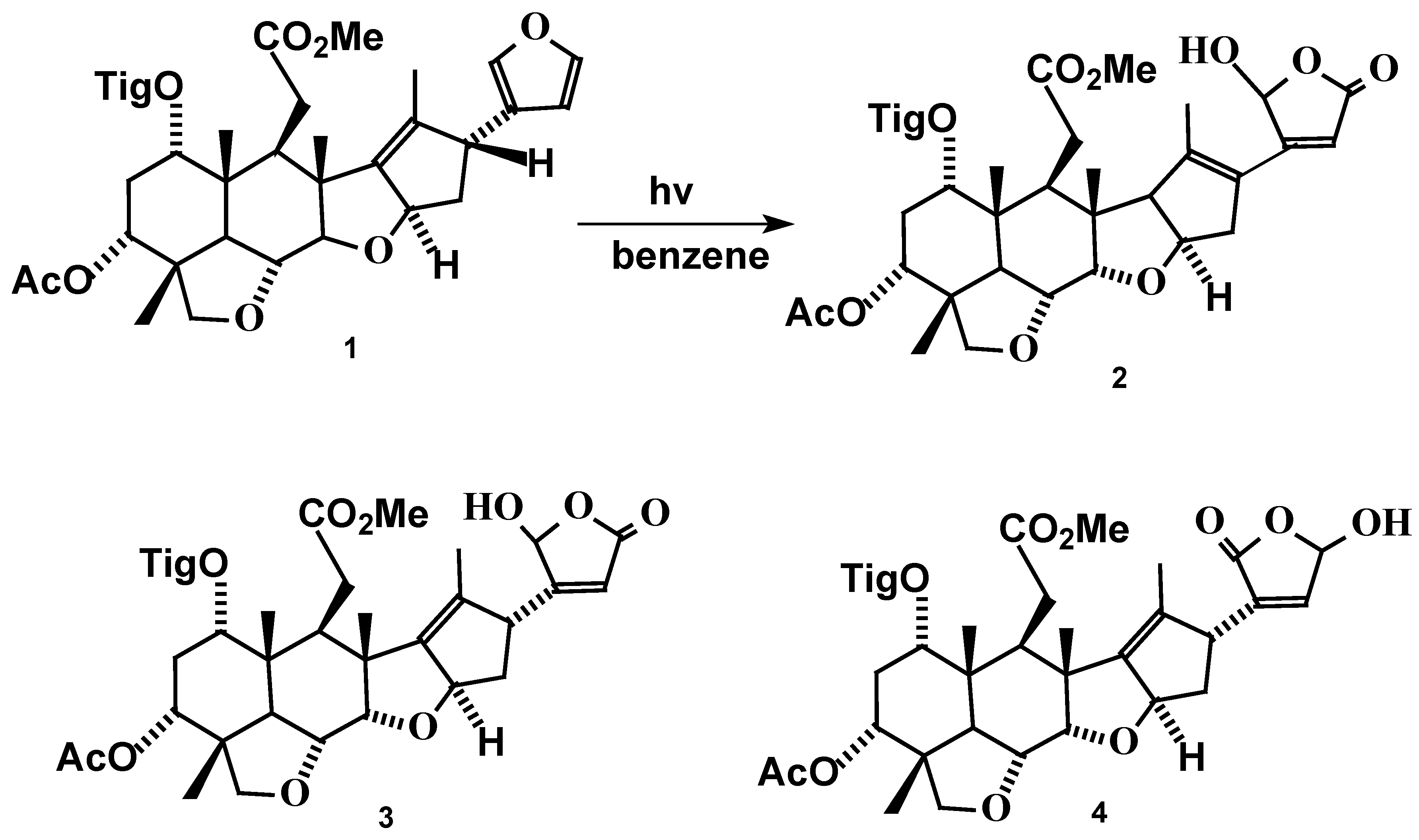
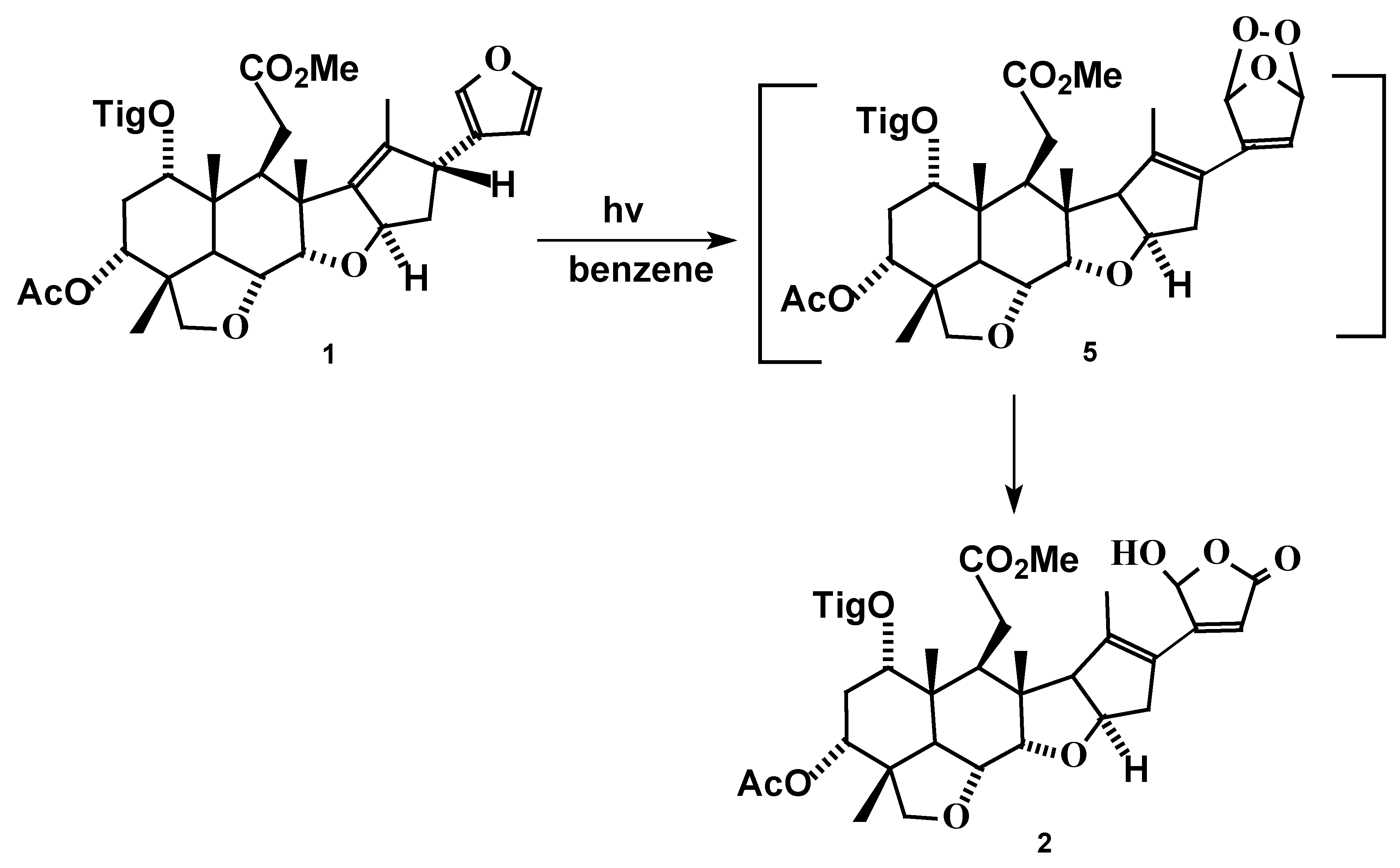
Results and Discussion
Acknowledgments
Experimental
General
Irradiation of Salannin (1).
Analytical and spectral data of compound 2.
References
- Kraus, W. The Neem Tree, Azadirachta indica A.Juss and other Meliaceous Plants; Schmutterer, H., Ed.; Verlag Chemie: Weinheim, 1995; pp. 35–38. [Google Scholar]
- Blaney, W.M.; Simmonds, M.S.J.; Ley, S.V.; Anderson, J.C.; Toogood, P.L. Antifeedant effects of Azadirachtin and structurally related compounds on lepidoptera larvae. Entomol. Exp. Appl. 1990, 55, 149–160. [Google Scholar] [CrossRef]
- Butterworth, J.H.; Morgan, E.D. Isolation of a substance that suppresses feeding in locust. J. Chem. Soc. Chem. Commun. 1968, 23–24. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Narasimhan, N.S.; Suresh, G.; Partho, P.D.; Geetha Gopalakrishnan. Insect antifeedant and growth regulating activities of salannin and other C-seco limonoids from neem oil in relation to azadirachtin. J. Chem. Ecol. 1996, 22, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, M.A.; Yamasaki, R.B.; Klocke, J.A. Biological activity of azadirachtin, three derivatives, and their ultraviolet radiation degradation products against tobacco bud worm (Lepidoptera: Noctuidae) larvae. J. Econ. Entomol. 1989, 82, 58–63. [Google Scholar] [CrossRef]
- Stokes, J.B.; Redfern, R.E. Effect of sunlight on azadirachtin: antifeeding potency. J. Environ. Sci. Health 1982, A17, 57–65. [Google Scholar] [CrossRef]
- Jarvis, A. P.; Johnson, S.; Morgan, E.D.; Simmonds, M.S.J.; Blaney, W.M. Photooxidation of nimbin and salannin-tetranortriterpenoid from neem tree. J. Chem. Ecol. 1997, 23, 2841–2860. [Google Scholar] [CrossRef]
- Henderson, R.; McCrindle, R.; Melera, A.; Overton, K.W. Tetranortriterpenoids-IX The constitution and stereochemistry of salannin. Tetrahedron 1968, 24, 1525–1528. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Suresh, G.; Geetha Gopalakrishnan. A direct preparative high performance liquid chromatography procedure for the isolation of major triterpenoids and their quantitative determination in neem oil. J. Liq. Chromatogra. 1995, 18, 3465–3471. [Google Scholar] [CrossRef]
- Geetha Goplakrishnan.; Pradeep Singh, N.D.; Kasinath, V.; Malathi, R.; Rajan, S.S. Photooxidation of cedrelone,a tetranortriterpenoid from Toona ciliata. Photochem. Photobio 2000, 72, 464–466. [Google Scholar]
- Foote, C.S.; Wuesthoff, M.T.; Wexler, S.; Burstain, I.G.; Denny, R.; Schenck, G.O.; Schulte-Elte, K-M. Photosensitized oxigenation of alkyl substituted furans. Tetrahedron 1967, 23, 2583–2599. [Google Scholar] [CrossRef]
- Gilbert, A. Photochemistry in Organic Synthesis; Coyle, J.D., Ed.; Royal Society of Chemistry: Cambridge, U.K., 1986; pp. 141–163. [Google Scholar]
- Pradeep Singh, N.D. Ph.D. Thesis, University of Madras, November 2000.
- Sample Availability: Both the starting compound (50 mg.) and the photoproducts (5 mg. each) are available from the author.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Gopalakrishnan, G.; Pradeep Singh, N.D.; Kasinath, V. Photomediated Transformation of Salannin, a Tetranortriterpenoid from Azadirachta indica A. Juss. Molecules 2001, 6, 551-556. https://doi.org/10.3390/60600551
Gopalakrishnan G, Pradeep Singh ND, Kasinath V. Photomediated Transformation of Salannin, a Tetranortriterpenoid from Azadirachta indica A. Juss. Molecules. 2001; 6(6):551-556. https://doi.org/10.3390/60600551
Chicago/Turabian StyleGopalakrishnan, Geetha, N. D. Pradeep Singh, and V. Kasinath. 2001. "Photomediated Transformation of Salannin, a Tetranortriterpenoid from Azadirachta indica A. Juss" Molecules 6, no. 6: 551-556. https://doi.org/10.3390/60600551



