Synthesis and Characterization of some New Mesoionic 1,3-Thiazolium-5-thiolates via Cyclodehydration and in situ 1,3-Dipolar Cycloaddition/Cycloreversion
Abstract
:Introduction

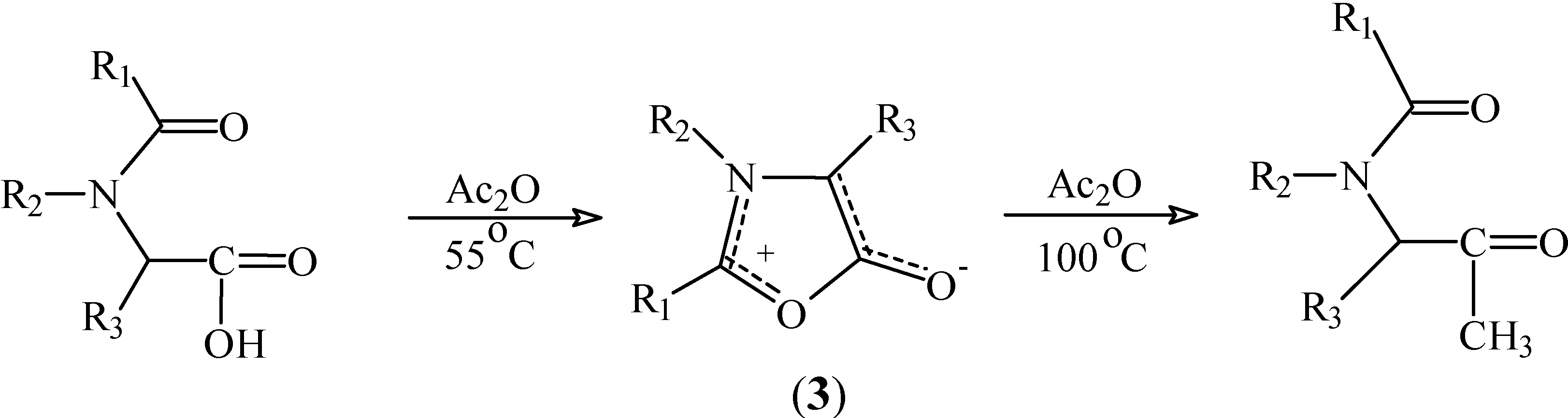
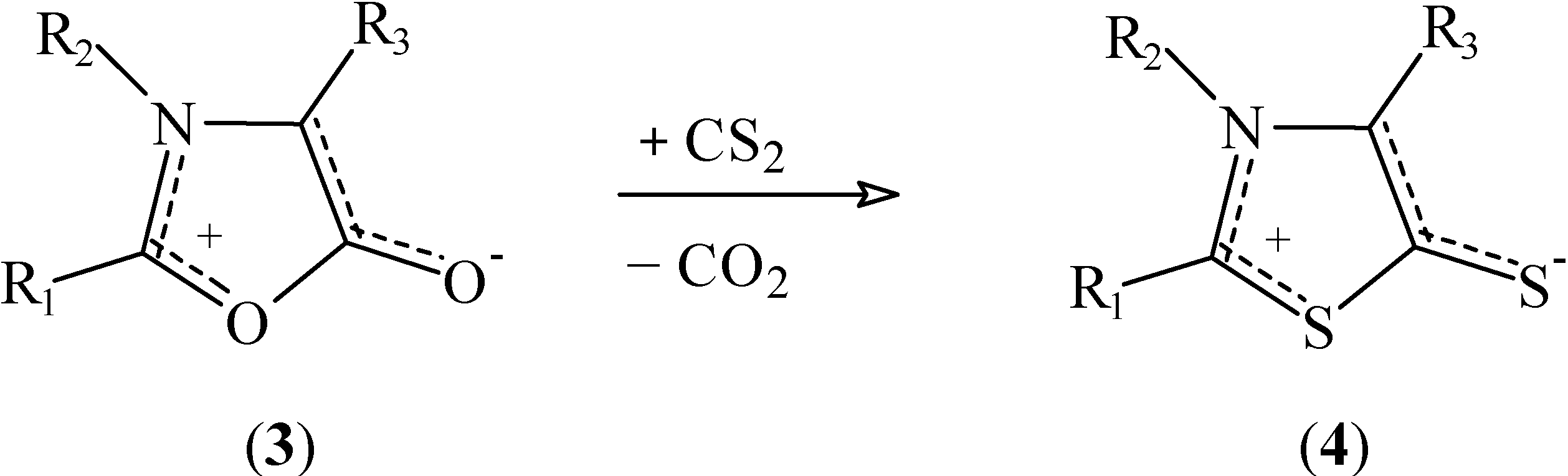
Results and Discussion
 |
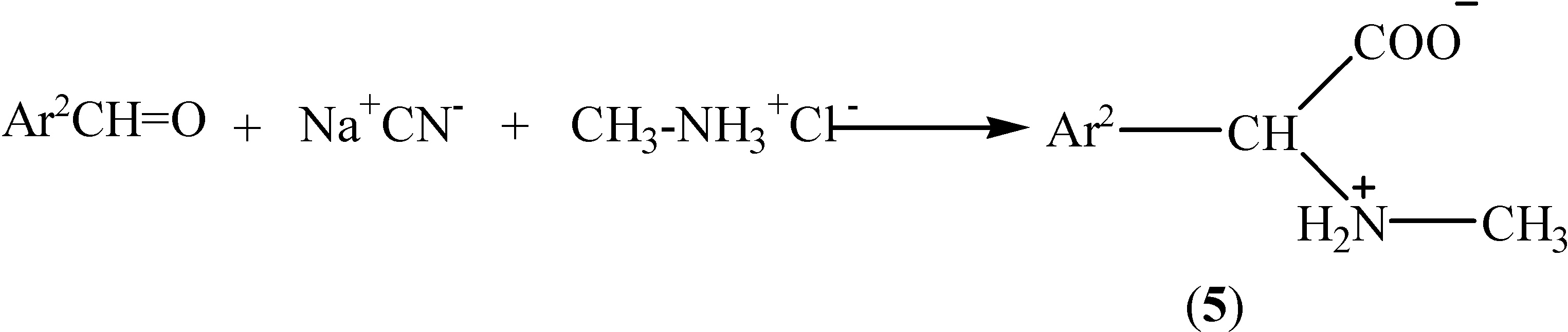
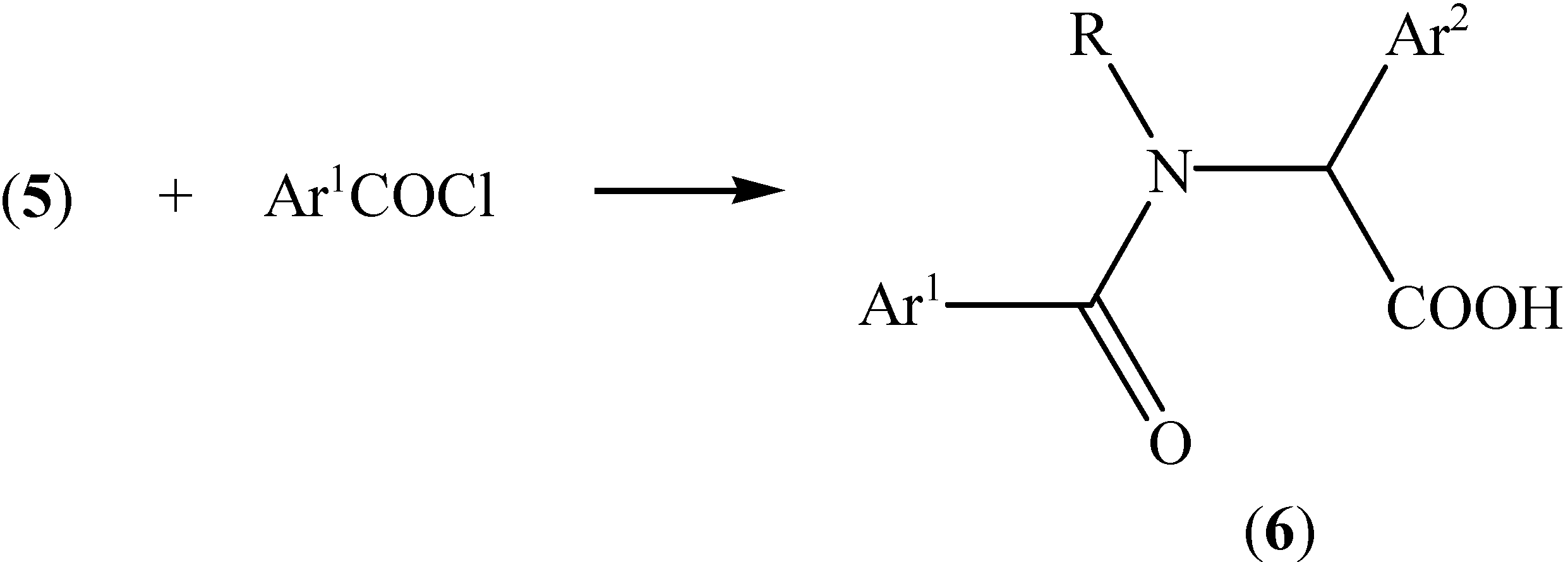

Conclusions
Acknowlegements
Experimental
General
Synthesis of mesoionic 1,3-thiazolium-5-thiolates (4)
Mesoionic 2-(p-chlorophenyl)-3-methyl-4-(p-tolyl)-1,3-thiazolium-5-thiolate (7a)
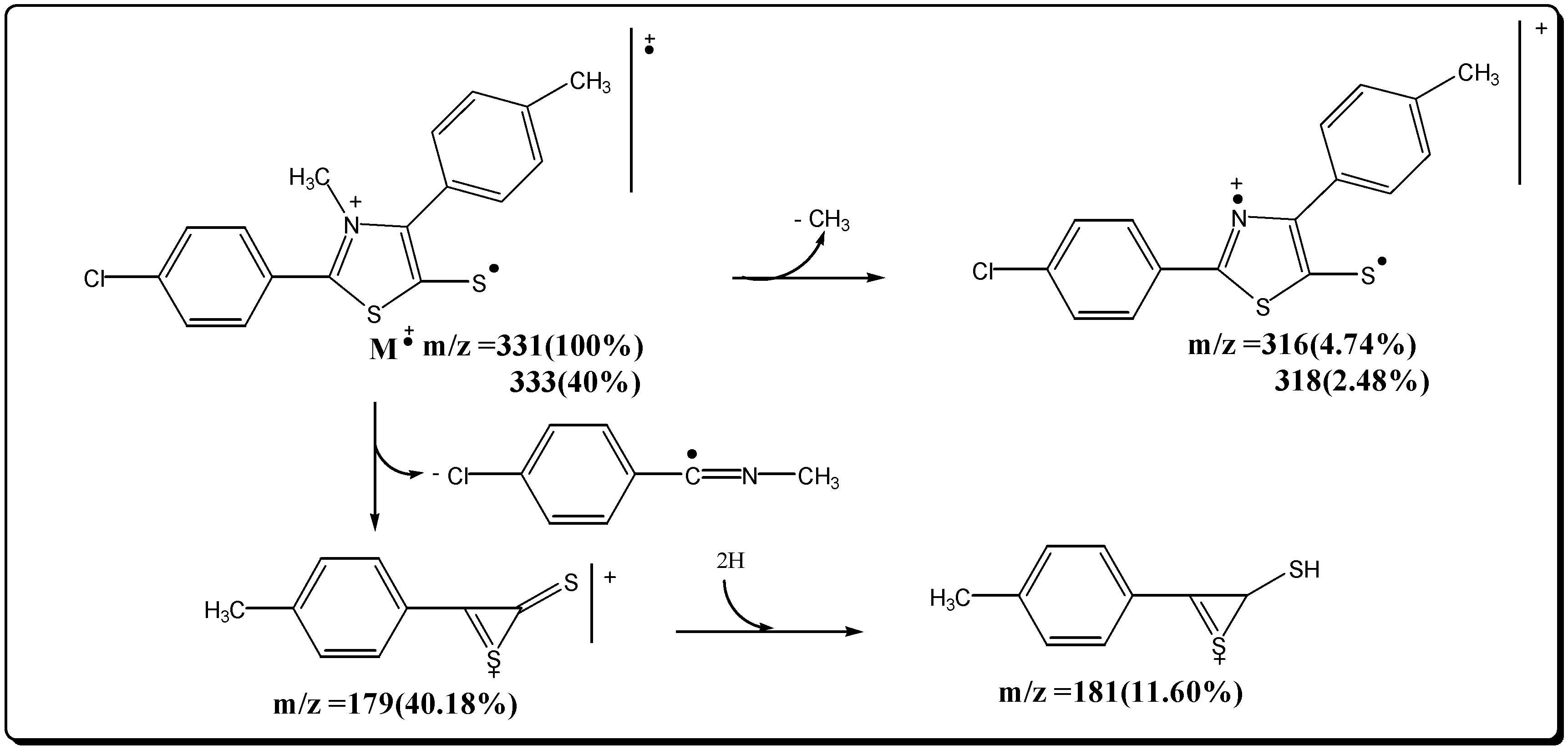
Mesoionic 2-(p-trifluorophenyl)-3-methyl-4-(p-tolyl )-1,3-thiazolium-5-thiolate (7b)
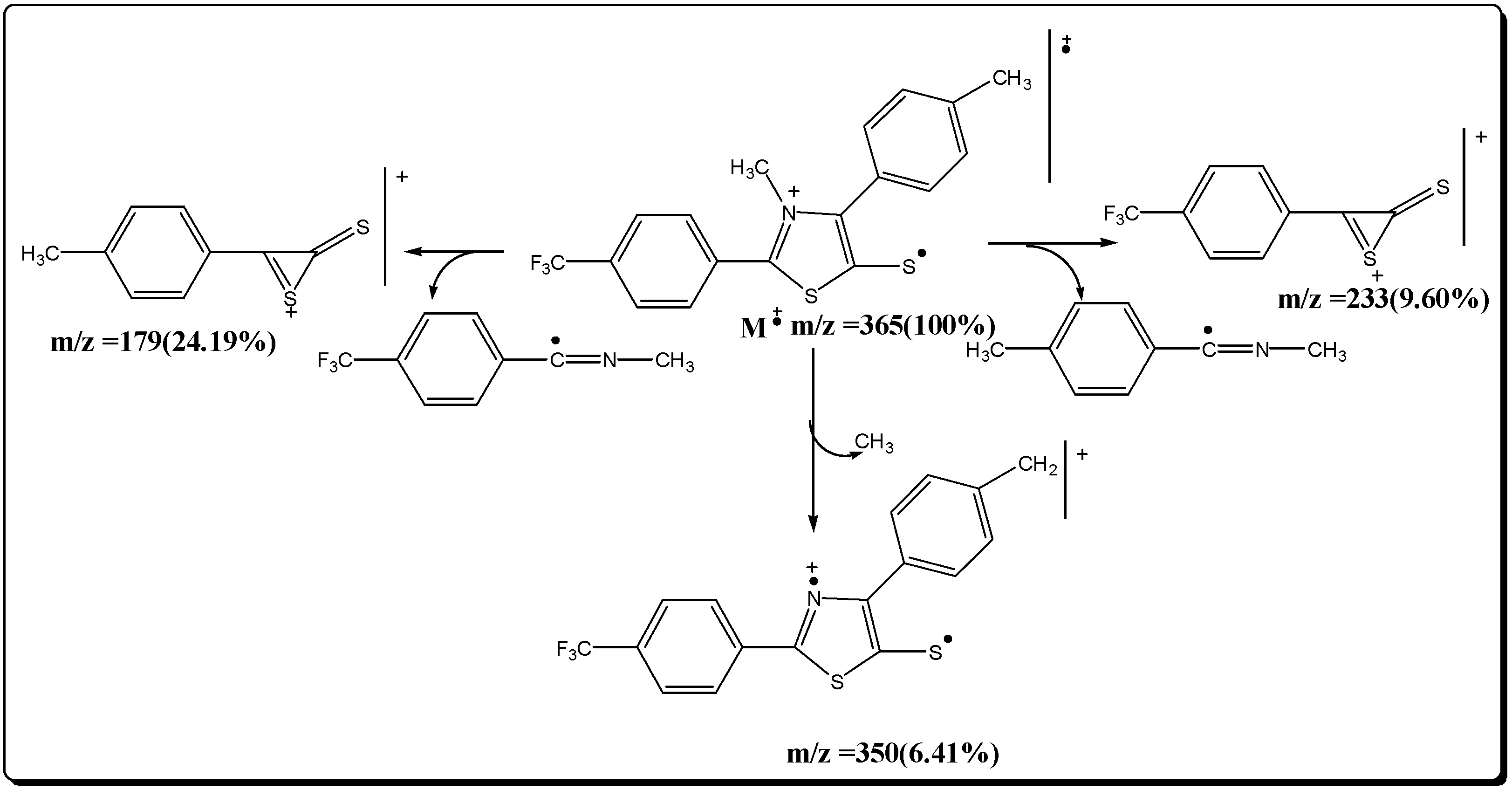
Mesoionic 2-(p-trifluorophenyl)-3-methyl-4-(p-isopropylphenyl)-1,3-thiazolium-5-thiolate (7c)
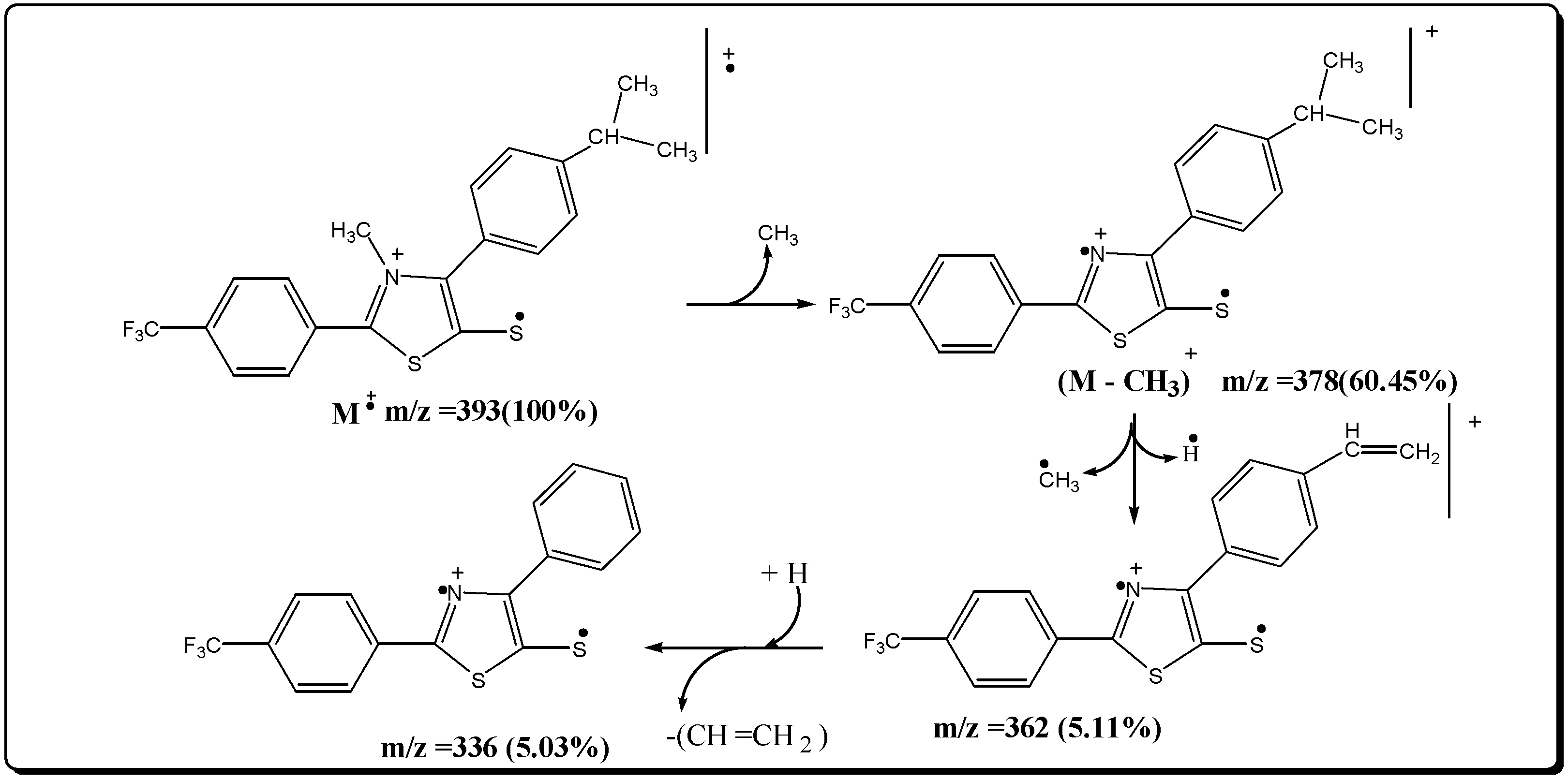
Mesoionic 2-(p-nitrophenyl)-3-methyl-4-(p-isopropylphenyl)-1,3-thiazolium-5-thiolate (7d)
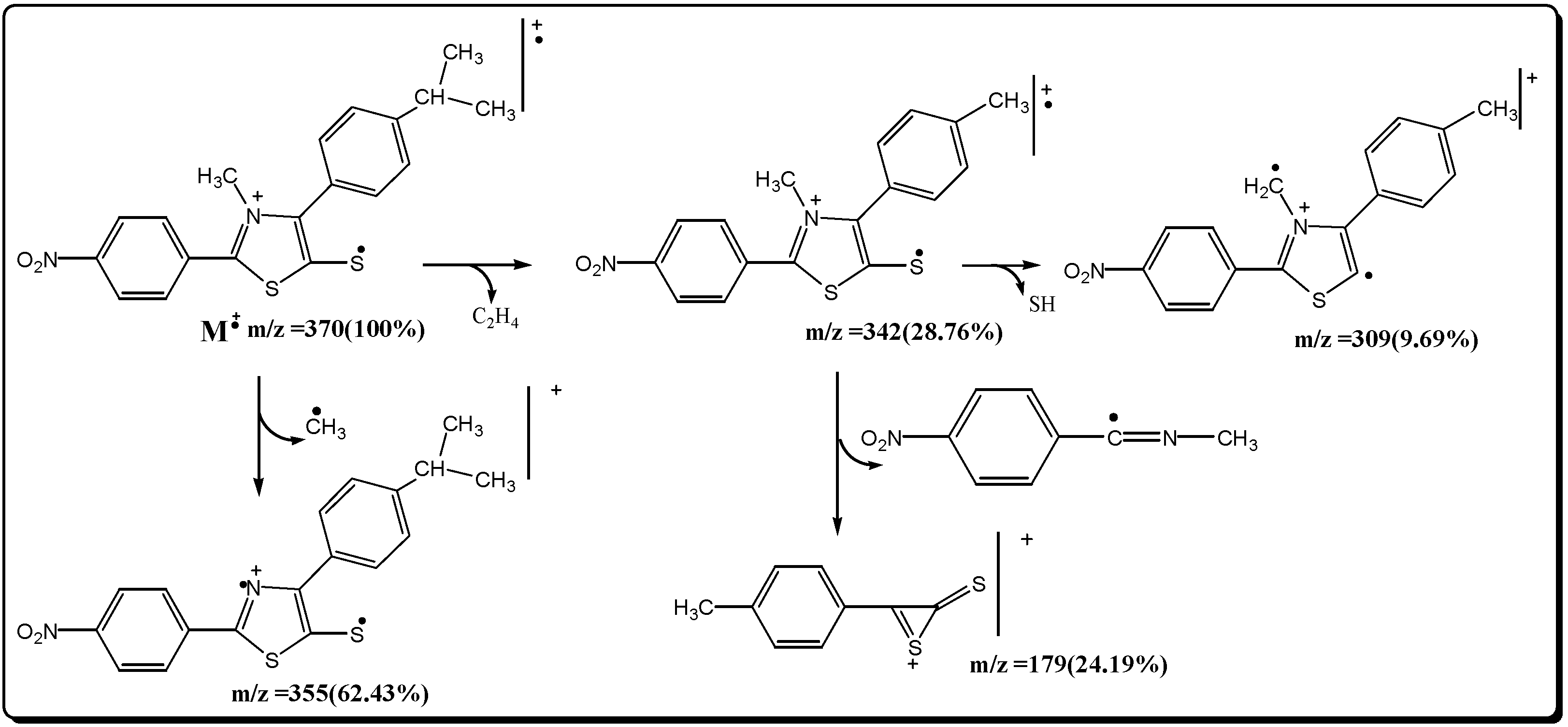
References
- Fischer, E.; Besthorn, E. Über die Hydrazinverbindungen. Ann 1882, 212, 316–339. [Google Scholar]
- Schönberg, A. Constitution and isomerism of certain triazole derivatives of the nitrone type in the light of the Bredt rule and the theory of resonance. J. Chem. Soc. 1938, 824–5. [Google Scholar] [CrossRef]
- Baker, W.; Ollis, W.D. Mesoionic Compounds. Quart. Rev. 1957, 11, 15–29. [Google Scholar] [CrossRef]
- Ollis, W.D.; Ramsden, C.A. Mesoionic Compounds. Adv. Heterocycl. Chem. 1976, 19, 3–122. [Google Scholar]
- Potts, K.T. Mesoionic ring systems, heterocycles of theoretical interest and synthetic potential. Lect. Heterocycl. Chem. 1978, 4, 35–46. [Google Scholar]
- Oliveira, M.B.; Miller, J.; Pereira, A.B.; Galembeck, S.E.; Moura, G.L.C.; Simas, A.M. Mesoionic 2-N-cycloalkyl-1,3-dithiolium-4-thiolates. Phosphorus, Sulfur, Silicon Relat. Elem. 1996, 108, 75–84. [Google Scholar] [CrossRef]
- Huisgen, R. Cycloaddition Reactions of Mesoionic Aromatic Compounds. Chem. Soc. Spec. Publ. 1967, 21, 51–73. [Google Scholar]
- Huisgen, R.; Funke, E.; Schaefer, F.C.; Gotthaerdt, H.; Brunn, E. Cycloadditions of mesoionic 5-oxazolone to some hetero-multiple bonds. Tetrahedron Lett. 1967, 19, 1809–1814. [Google Scholar] [CrossRef]
- (a) Newton, C.G.; Ramsden, C.A. Mesoionic heterocycles (1976–1980). Tetrahedron 1982, 38(20), 2964–3084. [Google Scholar]; (b) Barrett, G.C. The chemistry of 1,3-thiazolinone hydroxy-1,3-thiazole systems. Tetrahedron 1980, 36, 2023–2058. [Google Scholar]; (c) Ollis, W.D.; Stanforth, S.P. Tetrahedron 1985, 41, 2239–2329.
- Bayer, H.O.; Huisgen, R.; Knorr, R.; Schaefer, F.C. Preparation and properties of mesoionic oxazolones. Chem Ber. 1970, 103, 2581–2597. [Google Scholar] [CrossRef]
- Edward, J.T.; Sheffler, R.H. Preparation of Mesoionic Dipyrido[1,2-a:b’1’,2’-c]imidazolium-11-thiolates from the Binz-Marx Reaction. J. Org. Chem. 1985, 50, 4855–4861. [Google Scholar] [CrossRef]
- (a) Knorr, B.R.; Huisgen, R. Mechanism of the Dakin-West reaction. I. Reaction of secondary N-acylamino acids with acetic anhydride. Chem Ber. 1970, 103(8), 2598–2610. [Google Scholar]; (b) Potts, K.T.; Choundhury, D.R. Mesoionic Compounds. 43. Ring Annelation Utilizing the Isomeric anhydro-2- and 3-Hydroxythiazolo[2,3-b]benzothiazolium Hydroxide Mesoionic Systems. J. Org. Chem. 1978, 43, 2697–2700. [Google Scholar]
- Clerin, D.; Meyer, B.; Fleury, J.P.; Fritz, H. Mesoionic trifluoroacetylated 5-imino oxazolines: a re-examination of the structure using 13C NMR spectroscopy and cycloaddition reactions. Tetrahedron 1976, 32, 1055–1059. [Google Scholar]
- (a) Potts, K.T.; Chen, S.J.; Szmuszkovicz, J. Anhydro-2-Mercaptothiazolo[3,2-f]-phenanthridinium hydroxide, a mesoionic thiazole ring system containing exocyclic sulfur. J. Org. Chem. 1977, 42, 2525–2526. [Google Scholar]; (b) see also ref. 8
- Sample Availability: Not available
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Lira, B.F.; De Athayde Filho, P.F.; Miller, J.; Simas, A.M.; De Farias Dias, A.; Vieira, M.J. Synthesis and Characterization of some New Mesoionic 1,3-Thiazolium-5-thiolates via Cyclodehydration and in situ 1,3-Dipolar Cycloaddition/Cycloreversion. Molecules 2002, 7, 791-800. https://doi.org/10.3390/71100791
Lira BF, De Athayde Filho PF, Miller J, Simas AM, De Farias Dias A, Vieira MJ. Synthesis and Characterization of some New Mesoionic 1,3-Thiazolium-5-thiolates via Cyclodehydration and in situ 1,3-Dipolar Cycloaddition/Cycloreversion. Molecules. 2002; 7(11):791-800. https://doi.org/10.3390/71100791
Chicago/Turabian StyleLira, Bruno Freitas, Petrônio Filgueiras De Athayde Filho, Joseph Miller, Alfredo Mayall Simas, Aderson De Farias Dias, and Maria Joaquina Vieira. 2002. "Synthesis and Characterization of some New Mesoionic 1,3-Thiazolium-5-thiolates via Cyclodehydration and in situ 1,3-Dipolar Cycloaddition/Cycloreversion" Molecules 7, no. 11: 791-800. https://doi.org/10.3390/71100791




