Synthesis of Thieno[2,3-d]-1,3-dithiol-2-thiones from Thieno[2,3-d]-1,2,3-thiadiazoles: Matryoshka-type autoclave for high-temperature, high-pressure thermolysis microscale reactions
Abstract
:Introduction
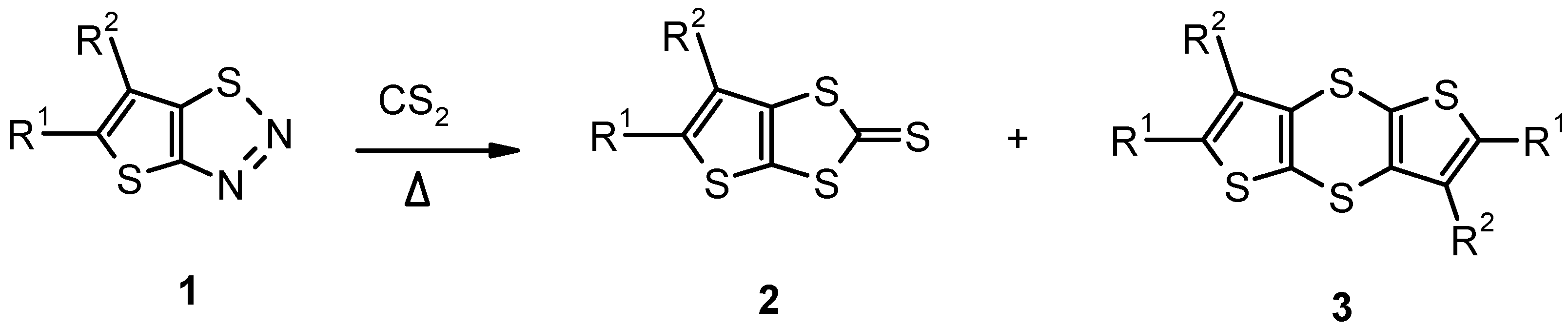
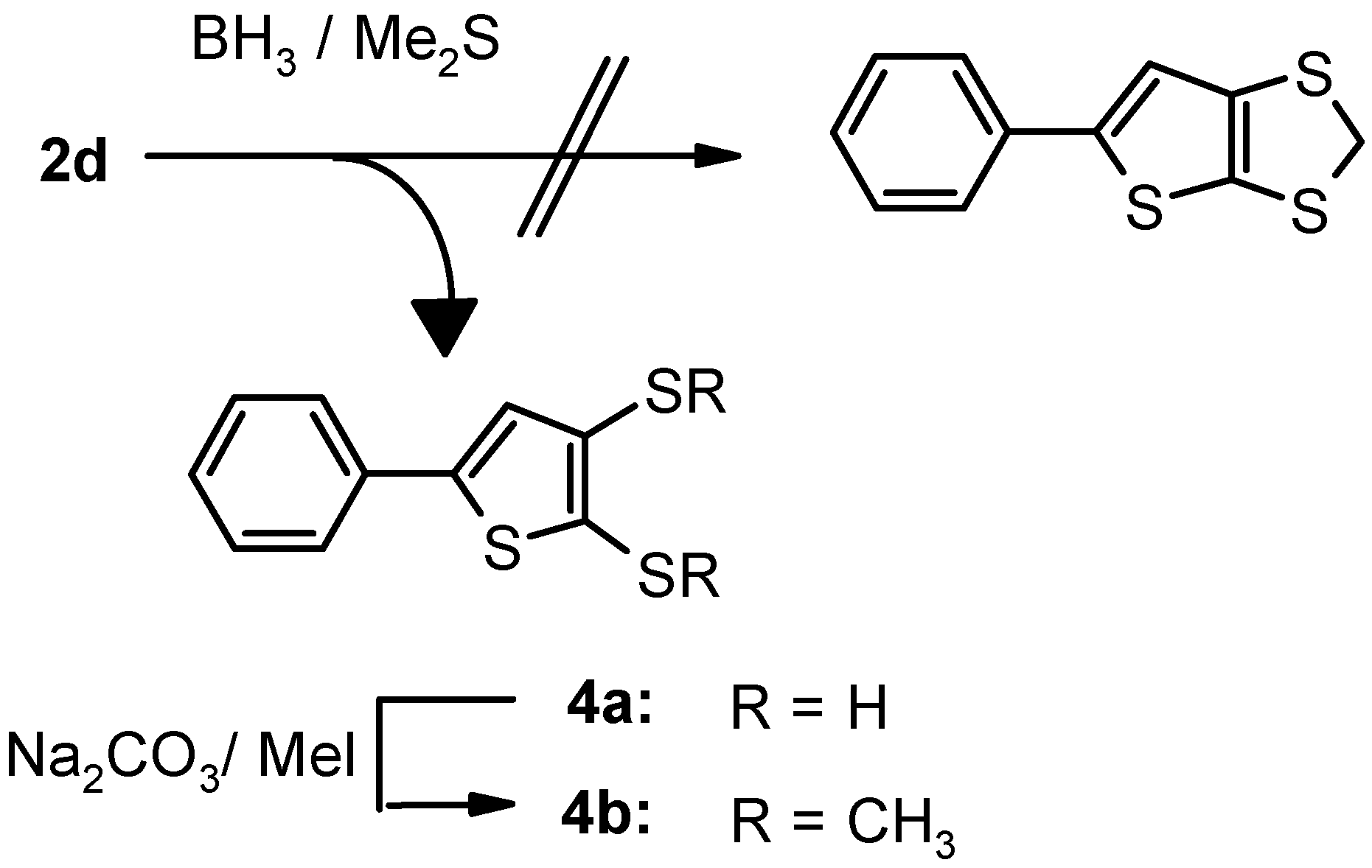
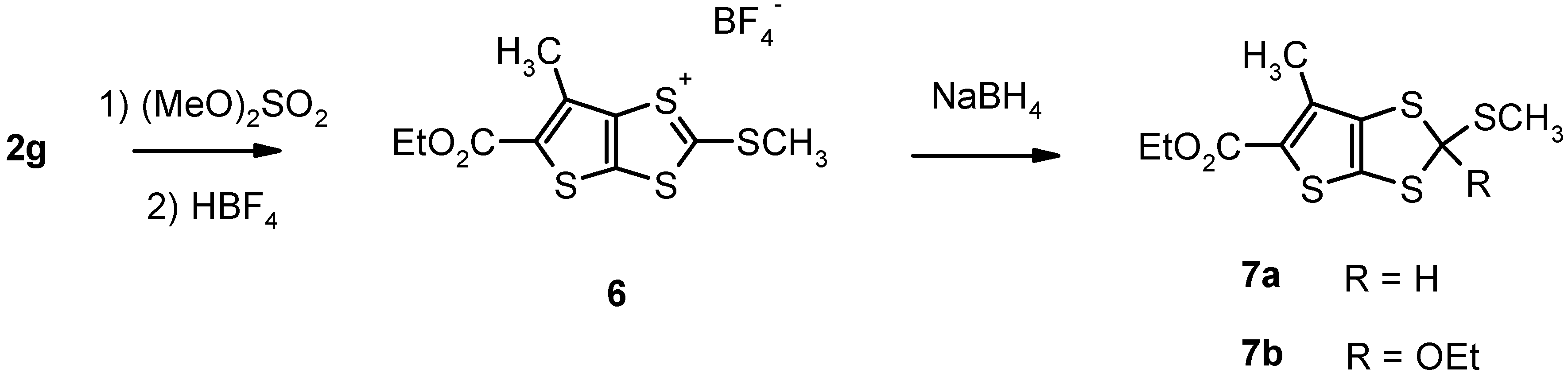
Results and Discussion

Conclusions
Experimental
General
| Run | Starting Mat. | R1 | R2 | Scale (mg) | Temp. (°C) | Time (h) | Product (yield, %) | m.p. (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | H | H | 750 | 210-220 | 7 | 2a (37) | 127-130 (toluene) |
| 2 | 1a | H | H | 240 | 230-240 | 8 | 2a (67) | 127-130 |
| 3 | 1b | Cl | H | 140 | 260-270 | 9 | 2b (10)a | 210-215 (LC) |
| 4 | 1c | CH3 | H | 140 | 235-245 | 9 | 2c (77)b | – |
| 5 | 1d | C6H5 | H | 150 | 240-267 | 9 | 3d (35) | 223-224 |
| 6 | 1d | C6H5 | H | 150 | 210-220 | 7 | 2d (80) | 175-177 |
| 7 | 1d | C6H5 | H | 840 | 210-230 | 6 | 2d (80) | 175-177 |
| 8 | 1e | 4-F-C6H4 | H | 140 | 230-240 | 9 | 2e (10) | 156-158 |
| 9 | 1f | C6H5 | CN | 610 | 210-220 | 9 | ||
| 3f (36) | 302-305 | |||||||
| 10 | 1g | COOC2H5 | CH3 | 8550 | 207-218 | 7 | 2g (29)c | 169-170 |
| 11 | 1g | COOC2H5 | CH3 | 3500 | 225-232 | 6 | 2g (54) | 169-170 |
| Product | Anal. | MS (m/z) | Remarks, NMR |
|---|---|---|---|
| 2a | Calcd for C5H2S4: C, 31.55; H, 1.06; Found: C, 31.70; H, 1.32. | 190 (M+) | 1H-NMR: δ 6.85 (d, 1H, 4-H), 7.45 (d, 1H, H-6). 13C-NMR δ 119.9 (C-6), 130.8 (C-5), 132.5 (C-6a), 138.0 (C-3a), 215.2 (C=S). |
| 2b | Calcd for C5HClS4: C, 26.72; H, 0.05. Found: C, 26.74; H, 0.30. | 224 (M+, 100), 180, 150, 148, 113, 104, 69. | 13C-NMR (CDCl3/d6-DMSO) δ 119.5 (C-6), 130.0, 136.1, 134.8 (C-3a, C-5, C-6a), 215.2 (C=S). |
| 2c | 204 (M+, 100), 169, 160, 140, 128, 113, 99, 85, 84, 71, 59, 57. | The crude product contained unreacted 1c and sulfur. A sample for MS was purified by flash-LC on silica using 7:3 petroleum ether/CH2Cl2 1H-NMR δ 2.58 (s, 3H, CH3), 6.6 (s, 1H, C-H). | |
| 2d | Calcd for C11H6S4: C, 49.59; H, 2.27. Found: C, 49.35; H, 2.18. | 266 (M+, 100), 222, 190, 165, 146, 133, 121, 106, 102. | |
| 2e | Calcd for C11H5FS4: C, 46.45; H, 1.77. Found: C, 46.48; H, 1.87. | 284 (M+, 100), 240, 208, 164, 139, 120. | 1H-NMR δ 7.10 (s, 1H, H-6), 7.10-7.20 (m, 2H, arom.), 7.40-7.60 (m, 2H, arom.). 13C-NMR δ 115.5 (C-6), 116.2 (C-o ArF), 127.5 (C-m ArF), 128.0 and 132.0 (C-3a and C-6a), 138.5 (C-p ArF), 145.5 (C-5), 164.0 (C-ipso ArF), 214.5 (C=S). |
| 2f | Calcd for C12H5NS4: C, 49.46; H, 1.73; N, 4.81. Found: C, 49.22; H, 1.90; N, 4.47. | 291 (M+, 100), 247, 227, 215, 171, 121. | |
| 2g | Calcd for C9H8O2S4: C, 39.11; H, 2.92; S, 46.40. Found: C, 38.98; H, 2.67; S, 47.33. | 276 (M+, 100) | 1H-NMR δ 1.45 (t, 3H, CH3CH2), 2.57 (s, 3H, CH3), 4.38 (q, 2H, OCH2). |
| 3d | Calcd for C20H12S4: C, 63.12; H, 3.18. Found: C, 63.05; H, 3.31. | 380 (M+, 100), 348, 303, 259, 190. | 1H-NMR δ 7.05 (s, 2H, 3H and 6-H), 7.25-7.50 (m, 10H, arom.). 13C-NMR δ 128.3, 128.5, 131.4, 131.5, 131.9, 132.0, 132.2, 133.6. |
| 3f | Calcd for C22H10N2S4: C, 61.37; H, 2.34; N, 6.51. Found: C, 60.81; H, 2.09; N, 6.11. | 430 (M+, 100), 398, 215, 121. |
References and Notes
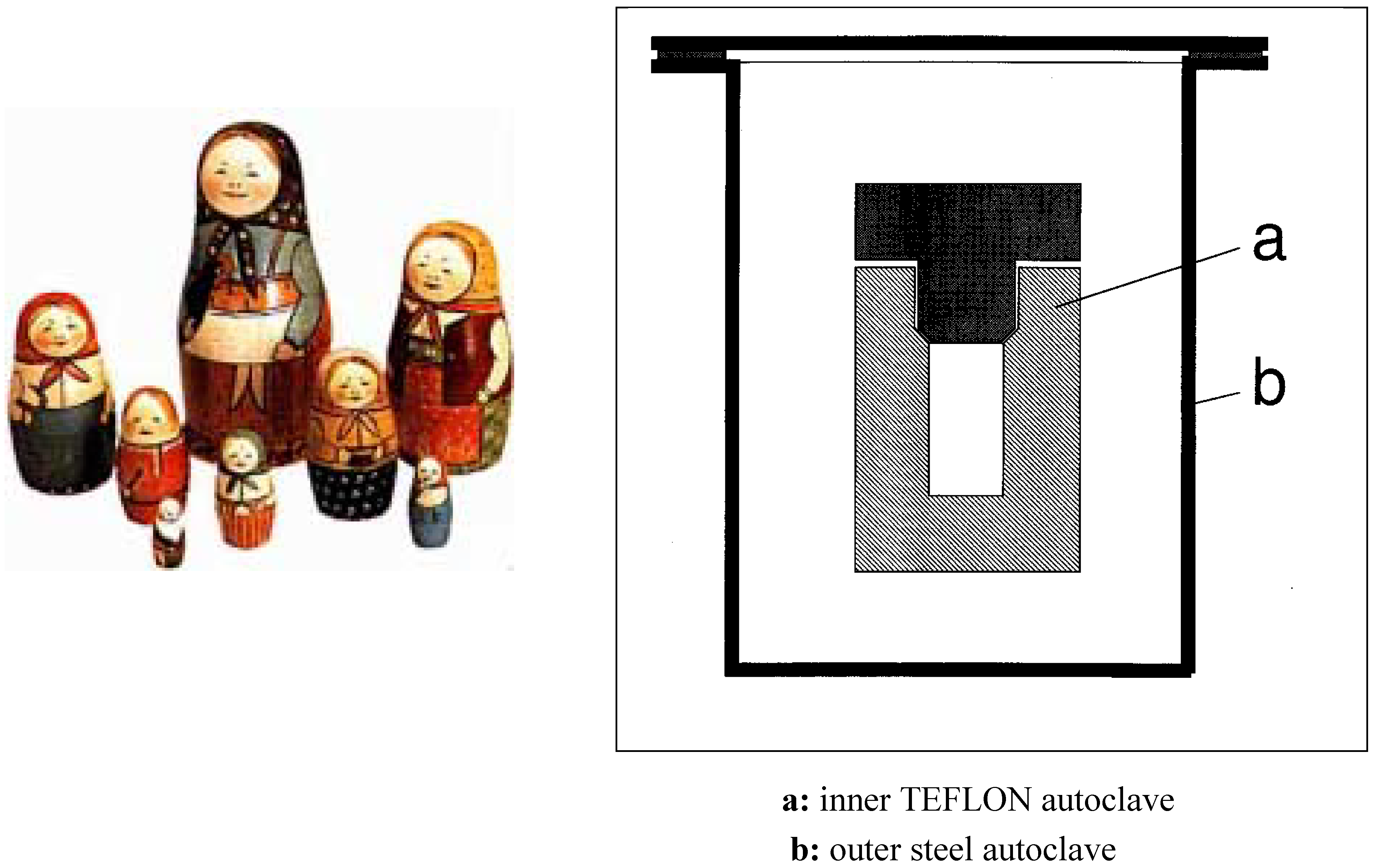
- Matryoshkas (=Matriushka, Matrushka) nesting dolls were introduced at the end of the 19th century by the Russian artist Sergei Maliutin who painted a wooden doll as a peasant girl with a rooster. Inside of this hollow doll there were seven more dolls all painted differently (Figure 1a).
- Bryce, M.R. Tetrathiafulfalene (TTF) and their selenium and tellurium analogs (TSF and TTeF): electron donors for organic metals. Aldrichimica Acta 1985, 18, 73–78. [Google Scholar]
- See e.g. Chinothionat: Büchel, K.H. (Ed.) Pflanzenschutz und Schädlingsbekämpfungsmittel; Georg Thieme Verlag: Stuttgart, 1977.
- Dölling, W.; Vogt, A.; Augustin, M. Synthesis of 1,3-dithiole-2-one derivatives, 2-ethylthio-1,3-dithiolium tetrafluoroborates and thieno[2,3-d]-1,3-dithiole-2-thiones. Z. Naturforsch. 1991, 46b, 133–138. [Google Scholar]
- Dölling, W.; Augustin, M.; Ihrke, R. A simple method for the preparation of 6-amino-thieno[2,3-d]-1,3-dithiol-2-thiones. Synthesis 1987, 655–657. [Google Scholar]
- Litvinov, V.P.; Dzhumaev, I.A. Condensed heteroaromatic 1,3-dithiole-2-thiones and their selenium analogs. Izv. Akad. Nauk SSSR, Ser. Khim. 1982, 717–718. [Google Scholar]
- Litvinov, V.P.; Dzhumaev, I.A.; Zolotarev, B.M. Synthesis and mass-spectrometric study of condensed thiones-precursors of thiophene-type heterofulvalenes. Izv. Akad. Nauk SSSR, Ser. Khim. 1983, 2105–10. [Google Scholar]
- Rasheed, K.; Warkentin, J.D. Thermal decomposition of dinitropyridyl and dinitrothienyl dithiocarbamates and t-butyl trithiocarbonates. J. Heterocycl. Chem. 1981, 18, 1581–1585. [Google Scholar]
- Engler, E.M.; Patel, V.V.; Andersen, J.R.; Schumaker, R.R.; Fukushima, A.A. Organic Metals. Systematic molecular modifications of hexamethylene tetraheterofulvalene donors. J. Am. Chem. Soc. 1978, 100, 3769–3776. [Google Scholar] Kumar, E.V.K.S.; Singh, J.D.; Singh, H.B.; Das, K.; Verghese, B. Synthesis of Some Functionalised Isomeric Bis(ethylendithio)tetrahydrothiafulvalene (BEDT.TTF) and Dithiophenetetrahydrofulvalene (DTTTF)-p-Donors. Tetrahedron 1997, 53, 11627–11644. [Google Scholar]
- Rovira, C.; Santalo, N.; Veciana, J. Bis(thiodimethylene)-tetrathiafulvalene (BTDM-TTF). A new π-electron donor with relevant oxidation properties. Tetrahedron Lett. 1989, 30, 7249–7252. [Google Scholar] Rovira, C.; Veciana, J.; Santalo, N.; Tarres, J.; Cirujeda, J.; Molins, E.; Llorca, J.; Espinosa, E. Synthesis of Several Isomeric Tetrathiafulvalene p-Electron Donors with Peripheral Sulfur Atoms. A Study of Their Radical Cations. J. Org. Chem. 1994, 59, 3307–3313. [Google Scholar]
- Jordis, U.; Rudolf, M. Conversion of cyclic trithiocarbonates to thioacetals, including 1,3-dithiane, by reduction with diisobutylaluminium hydride (DIBAL). Phosph. Sulf. 1984, 19, 279–283. [Google Scholar]
- Leading references for the preparation of thieno[2,3-d]-1,2,3-thiadiazoles: Stanetty, P.; Gorner, E.; Mihovilovic, M.D. An improved synthetic approach to thieno[2,3-d]-1,2,3-thiadiazole-carboxylates via diazotization of aminothiophene derivatives. J. Heterocycl. Chem. 1999, 36, 761–765. [Google Scholar] Stanetty, P.; Mihovilovic, M.D. A synthetic approach to methyl thieno[2,3-d]-[1,2,3]thiadiazolecarboxylates via diazotization. Monatsh. Chem. 1999, 130, 573–580. [Google Scholar] Stanetty, P.; Gorner, E.; Mihovilovic, M.D. An improved synthetic approach to thieno[2,3-d]-1,2,3-thiadiazolecarboxylates via diazotization of aminothiophene derivatives. J. Heterocycl. Chem. 1999, 36, 761–765. [Google Scholar]
- U. Jordis, unpublished results.
- Huisgen, R.; Weberndörfer, V. 1,3-Dipolare Additionen der Thioketocarbene. Experientia 1961, 17, 566. [Google Scholar]
- Jordis, U. Hydride reduction of 1,3-benzodithiole-2-thiones and -selones. J. Chem. Res. (S) 1986, 432, J. Chem. Res. (M) 1986, 3401. [Google Scholar]
- Bryce, M. R.; Coffin, M. A. J. Org. Chem. 1992, 57, 1696–1699.
- Brown, C. A.; Miller, R. D.; Lindsay, C. M.; Smith, K. Generation of 2-lithio-(2-methylthio)-1,3-benzodithioles, new carbonyl carbanion equivalents, and their application to the synthesis of unsymmetrical hexaorthooxalates. Tetrahedron Lett. 1984, 25, 991–994. [Google Scholar]
- Nakayama, J. The chemistry of 2-alkoxy-1,3-benzodithioles and 1,3-bonzodithiolium salts. Reactions and synthetic applications. Sulfur Rep. 1985, 4, 159–194. [Google Scholar]
- Aldoshina, M.Z.; Atovmyan, L.O.; Goldenberg, L.M.; Krasochka, O.N.; Lubovskaya, R.N.; Lubovskii, R.B.; Merzhanov, V.A.; Khidekel, M.L. A new series of radical-cation salts based on asymmetrical ethylendithiodimethyltetrathiafulvalene (EDTDM-TTF). J. Chem. Soc., Chem. Commun. 1985, 1658–1661. [Google Scholar]
- Simchen, G. Eine neue allgemeine Heterocyclensynthese durch Alkylierung von Nitril-Halogenwasserstoffaddukten. Habilitationsschrift Universität Stuttgart. 1968. [Google Scholar]
- Hennecke, H. DBP 1023464 (1958); [Chem. Abstr. 1960, 54, 5704e].
- Gewald, K.; Hain, U.; Madlenscha, M. 2,3-Heterocondensed thiophenes from substituted 2-aminothiophen-3-thiole. J. prakt. Chem. 1988, 330, 866–872. [Google Scholar]
- Gewald, K.; Hentschel, M.; Heikel, R. 2-Amino-thieno[2,3-d]thiazoles und 3-amino-thieno[2,3-c]isothiazoles. J. prakt. Chem. 1973, 315, 539–548. [Google Scholar]
- Sample Availability: Compounds 2c-d, 3d and 4b are available from MDPI.
Appendix: Information on Matryoshkas

© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Jordis, U.; Bhattacharya, K.; Boamah, P.Y.; Lee, V.J. Synthesis of Thieno[2,3-d]-1,3-dithiol-2-thiones from Thieno[2,3-d]-1,2,3-thiadiazoles: Matryoshka-type autoclave for high-temperature, high-pressure thermolysis microscale reactions. Molecules 2002, 7, 145-154. https://doi.org/10.3390/70200145
Jordis U, Bhattacharya K, Boamah PY, Lee VJ. Synthesis of Thieno[2,3-d]-1,3-dithiol-2-thiones from Thieno[2,3-d]-1,2,3-thiadiazoles: Matryoshka-type autoclave for high-temperature, high-pressure thermolysis microscale reactions. Molecules. 2002; 7(2):145-154. https://doi.org/10.3390/70200145
Chicago/Turabian StyleJordis, Ulrich, Kaberi Bhattacharya, Philip Y. Boamah, and Ving J. Lee. 2002. "Synthesis of Thieno[2,3-d]-1,3-dithiol-2-thiones from Thieno[2,3-d]-1,2,3-thiadiazoles: Matryoshka-type autoclave for high-temperature, high-pressure thermolysis microscale reactions" Molecules 7, no. 2: 145-154. https://doi.org/10.3390/70200145




