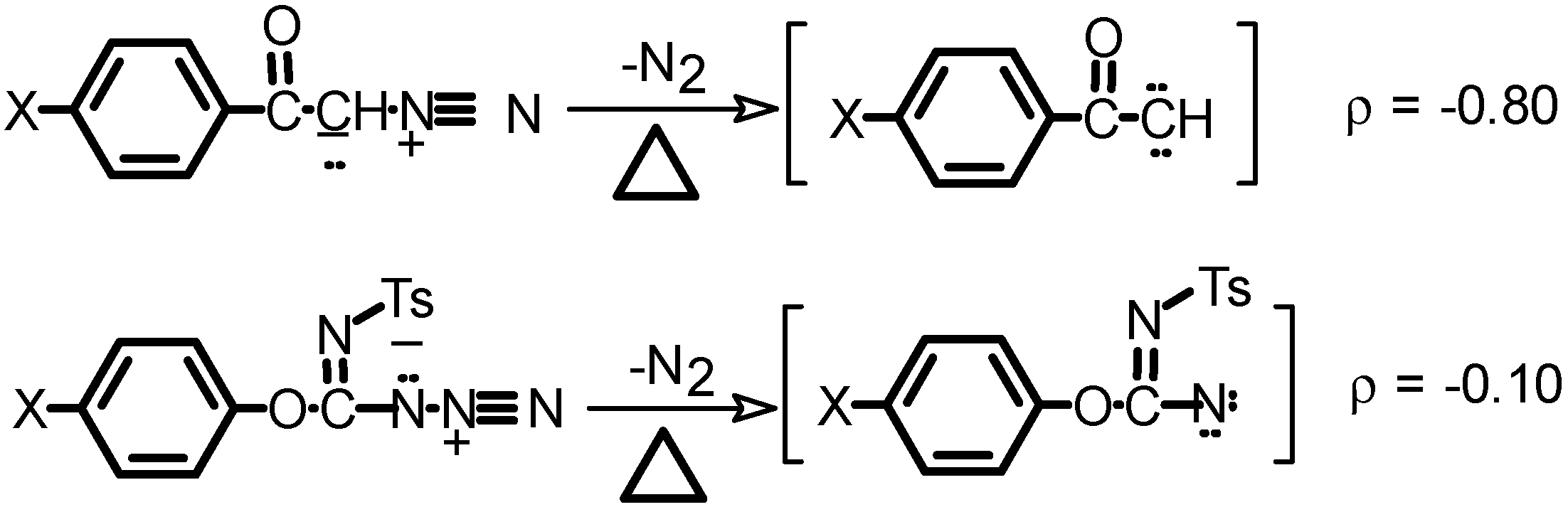
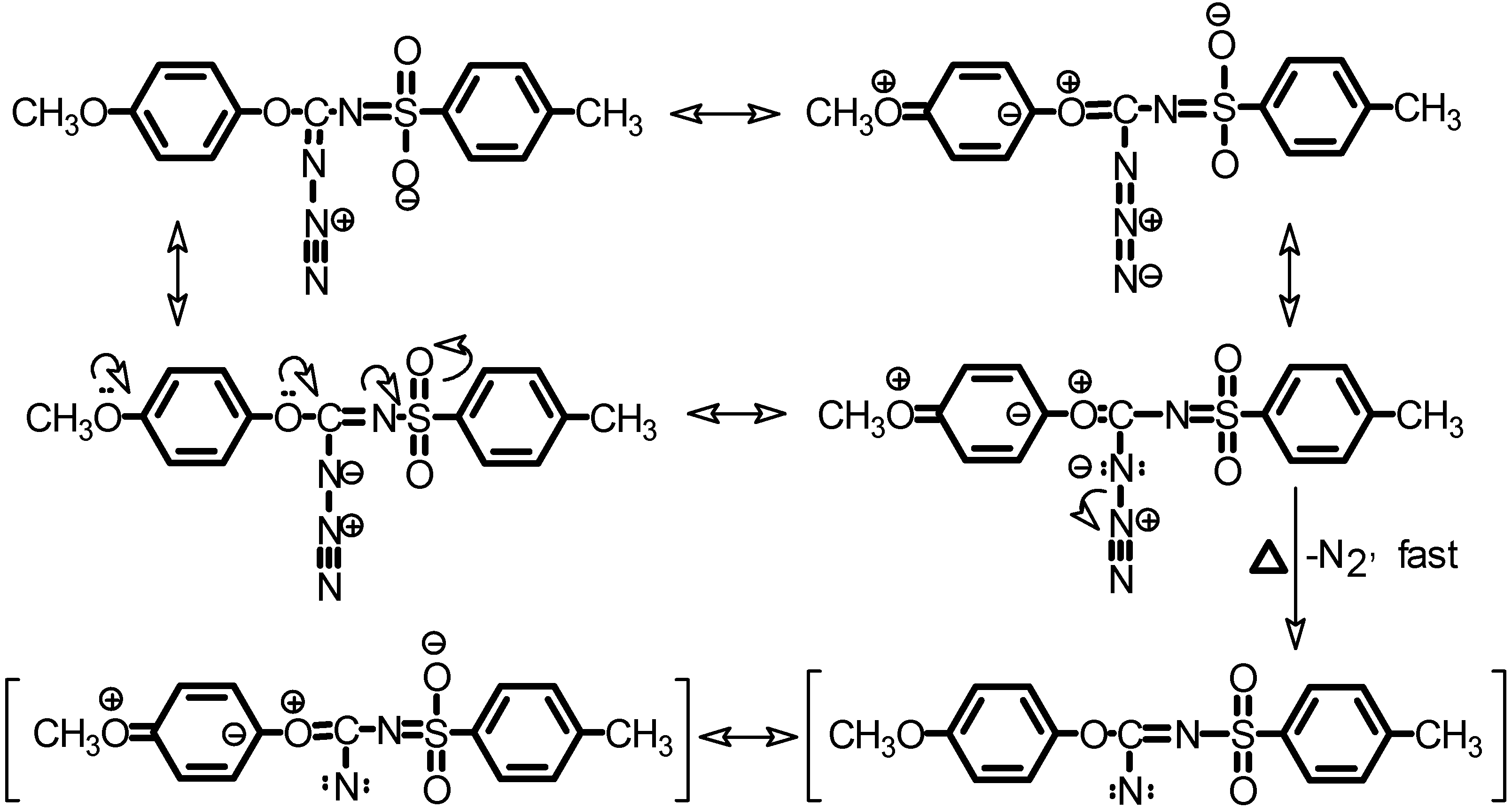
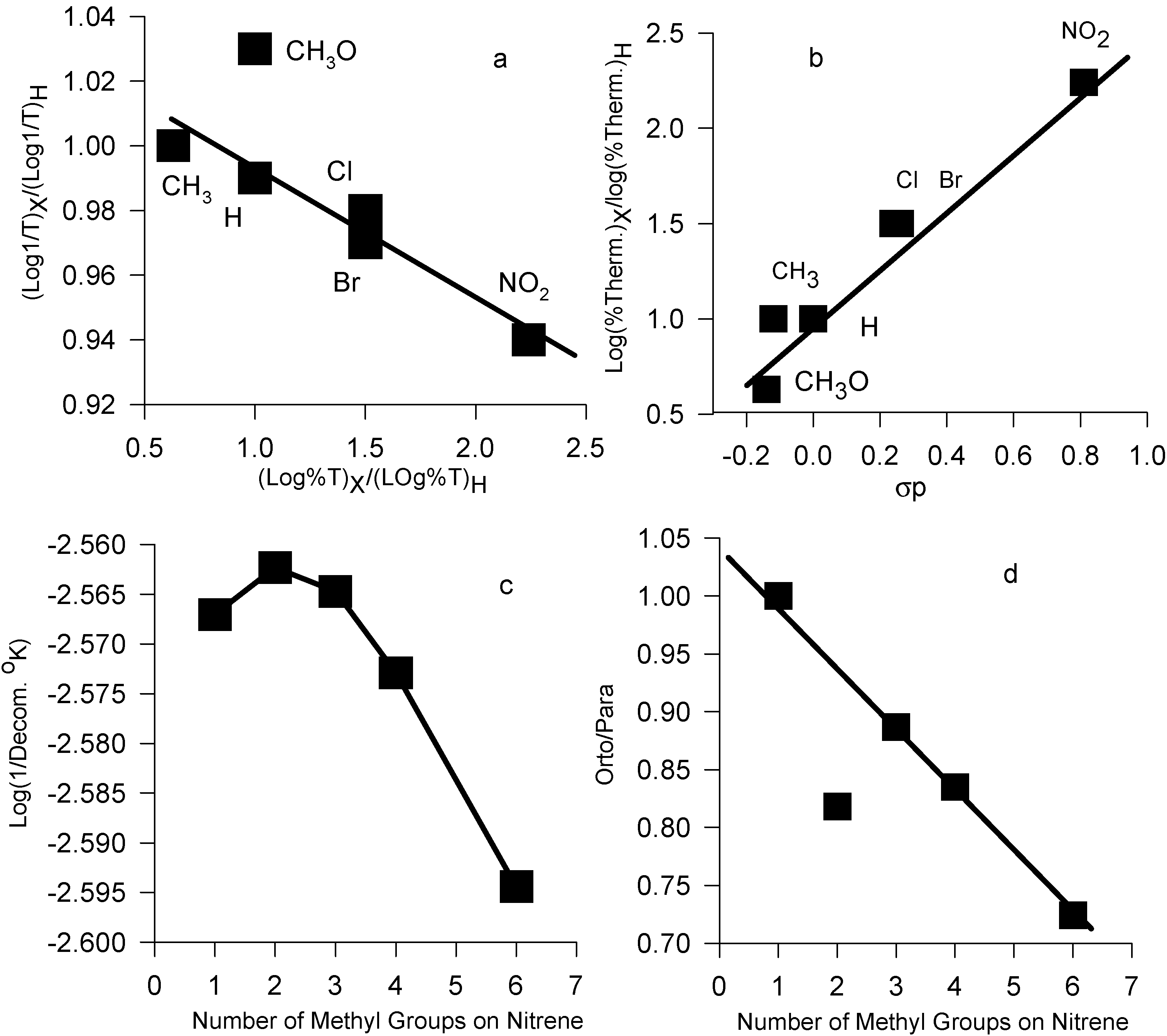
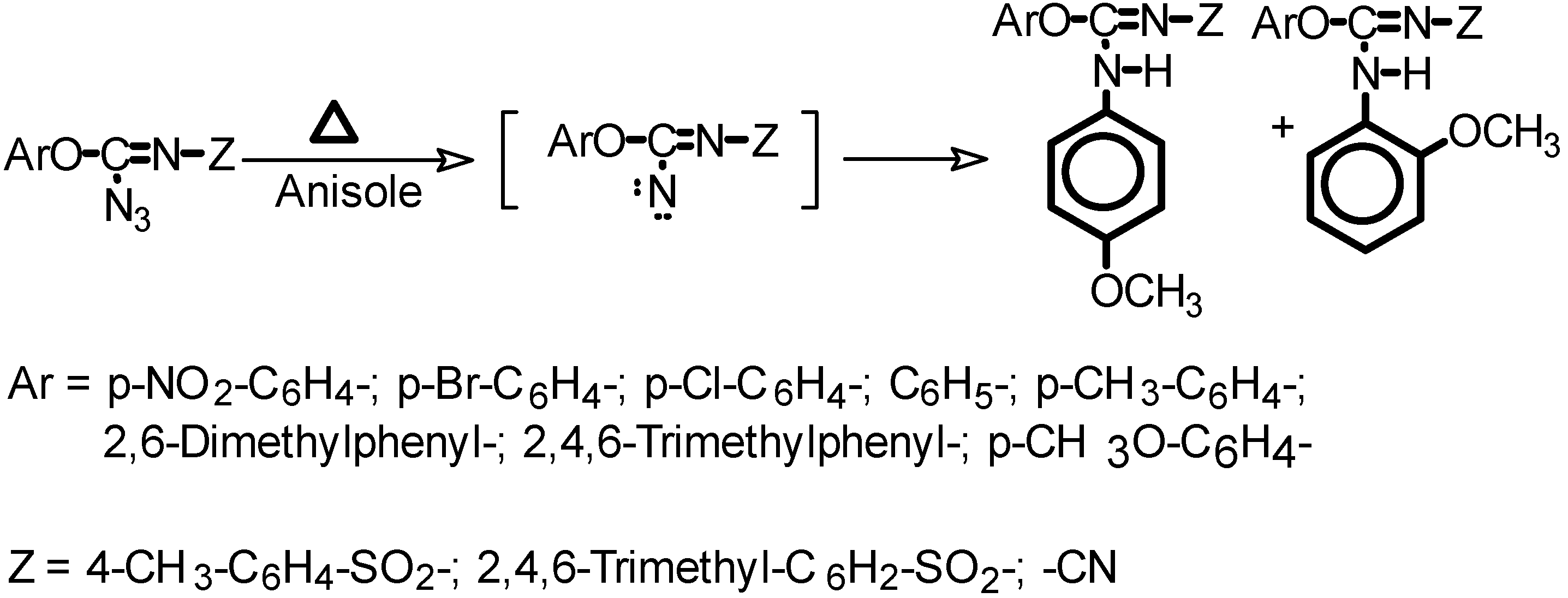Typical thermal decomposition of azides in anisole
Unless stated otherwise, azides were thermolyzed in a 70-fold excess of anisole in a 10±5 oC oil bath for 3 hrs. The progress of the reactions was followed by thin layer chromatography. Excess anisole was removed at 2-25 mmHg and 25-40 oC. The residue was analyzed by 1H-NMR, using TMS (tetramethylsilane) as an internal standard and, unless stated otherwise, CD3CN-CCl4 as solvent. The residue was either chromatographed on silica gel (60-200 mesh) or extracted in a Soxhlet extractor using a suitable solvent.
N'-(p-toluenesulfonylphenoxycarbimidoyl azide (1): Thermolysis of azide 1 (1.575 g, 38.5 mmol) was decomposed completely at 100 ±5 oC in the presence of anisole (38.5 mL) during 3 hrs. Reaction progress was followed by the visual color change of the solution (a colorless solution turned to light brown) and thin layer chromatography (TLC). Removal of the volatiles by vacuum distillation afforded 1.85 g (94%) of crude product mixture containg 6% Thermolyzate 1X, by weight. 1H-NMR (crude): δ ppm 2.40 (s, 3H, CH3); 3.80 (s, 3H, OCH3); 3.96 (s, 3H, OCH3); 6.80-7.90 (m, 13H, aromatic); 9.30 (s, 1H, NH); 9.80(s, 1H, NH). This crude mixture was washed with cold methanol to give N-4-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(phenoxy)isourea (1P): 1H-NMR δ ppm 2.50 (s, 3H, CH3); 3.90 (s, 3H, OCH3); 7.0-8.0, (m, 13H, aromatics); 9.50 (s, 1H, NH). The methanol soluble fraction gave mostly N-2-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(phenoxy)isourea (1O): 1H-NMR δ ppm 2.50 (s, 3H, CH3); 4.1 (s, 3H, OCH3); 7.0-7.80 (m, 13H, aromatics); 10.10 (s, 1H, NH).
N'-(p-toluenesulfonyl) (4-methylphenoxy)carbimidoyl azide (2): Thermolysis of azide 2 (2.31 g, 7 mmol) in anisole (54 mL) gave 2.6 g of product (90% yield) with 4% Thermolyzate 2X: 1H-NMR δ ppm 2.40 (s, 3H, CH3), 2.53 (s, 3H, CH3), 3.75 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 6.80-7.8 (m, 12H, aromatics). The crude mixture was washed with cold methanol to produce N-4-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(4-methylphenoxy)isourea (2P): 1H-NMR δ ppm 2.40 (s, 3H, CH3), 2.48 (s, 3H., CH3), 3.85 (s, 3H, OCH3), 6.90 (d, J = 7.5 Hz, 2H, aromatics), 7.05 (s, 4H, aromatics), 7.30 (d, J = 7.5 Hz, 2H, aromatics), 7.45 (d, J = 7.5 Hz, 2H, aromatics), 7.85 (d, J = 7.5 Hz, 2H, aromatics), 9.50 (s, 1H, NH). The methanol soluble fraction was concentrated under reduced pressure and crystallized from petroleum ether gave N-2-(methoxy-phenyl)-N'-(4-methylphenylsulfonyl)-O-(4-methylphenoxy)isourea (2O): 1H-NMR (CCl4-CDCl3): δ ppm 2.40 (s, 3H, CH3), 2.50 (s, 3H, CH3), 4.10 (s, 3H, OCH3), 7.0-7.90 (m, 12H, aromatics), 10 (s, 1H. NH).
N'-(p-toluenesulfonyl)(2,6-dimethylphenoxy)carbimidoyl azide (3): Thermolysis of azide 3 (2.064 g, 6 mmol) in anisole (46 mL) gave 2.54 g of crude mixture with 3% Thermolyzate 3X: 1H-NMR (CCl4-CDCl3): δ ppm 2.0 (s, 6H, CH3), 2.40 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 4.0 (s, 3H, OCH3), 6.85-7.85 (m, 11H, aromatics), 9.45 (s, 1H, NH), 10.05 (s, 1H, NH). This mixture was washed with cold methanol. The insoluble fraction gave N-4-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(2,6-dimethylphenoxy)-isourea (3P): 1H-NMR (CCl4-CDCl3): δ ppm 1.95 (s, 6H, CH3), 2.40 (s, 3H, CH3), 3.95 (s, 3H, OCH3), 6.80-7.0 (m 3H, aromatics), 6.90 (s, 4H, aromatics), 7.15 (d, j = 9 Hz, 2H), 7.60 (d, j = 9 Hz, 2H), 9.85 (s, 1H, NH). The methanol solution was condensed under reduced pressure to give N-2-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(phenoxy)isourea (3O): 1H-NMR (CCl4-CDCl3): δ ppm 2.0 (s, 6H, CH3), 2.40 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.85-7.85 (m, 11H, aromatics), 10.05 (s, 1H, NH).
N'-(p-toluenesulfonyl)(4-methoxyphenoxy)carbimidoyl azide (4). Thermolysis of 1.038 g (3 mmol) of azide 4 in anisole (33 mL) produced 1.20 g of crude mixture with 6% Thermolyzate 4X. The crude 1H-NMR (CCl4-pyridine-d6) δ ppm 2.40 (s, 3H, CH3), 3.80, (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 4.0 (s, 3H, OCH3), 6.70-7.90 (m, 12H, aromatics), 9.45 (s, 1H, NH), 10.05 (s, 1H, NH). Attempts to separate N-4-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(4-methoxyphenoxy)-isourea (4P) from N-2-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(4-methoxyphenoxy) isourea (4O) were unsuccessful.
N'-(p-toluenesulfonyl)(4-chlorophenoxy)carbimidoyl azide (5). Thermolysis of 1.75 g (5 mmol) of azide 5 in anisole (38 mL) gave 1.90 g of a crude mixture with 9% Thermolyzate 5X.1H-NMR δ ppm 2.40 (s, 3H, CH3), 3.78 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 6.80-7.80 (m, 12H, aromatics), 9.25 (s, 1H, NH), 9.70 (s, 1H, NH). All attempts to separate N-4-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(4-chloro-phenoxy)isourea (5P) from N-2-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(4-chlorophenoxy)-isourea (5O) failed.
N'-(p-toluenesulfonyl) (4-bromophenoxy)carbimidoyl azide (6). Thermolysis of 4.40 g (7.1 mmol) of azide 6 in anisole (24 mL) gave a crude product with 9% Thermolyzate 6X. 1H-NMR (CCl4-CDCl3): δ ppm 2.40 (s, 3H, CH3), 3.83 (s, 3H, OCH3), 4.0 (s, 3H, OCH3), 6.90-7.80 (m, 12H, aromatics), 9.46 (s, 1H, NH), 9.90 (s, 1H, NH). All attempts to separate the N-4-(methoxyphenyl)-N'-(4-methyl-phenylsulfonyl)-O-(4-bromophenoxy)isourea (6P) from N-2-(methoxyphenyl)-N'-(4-methylphenyl-sulfonyl)-O-(4-bromophenoxy)isourea (6O) failed.
N'-(p-toluenesulfonyl) (4-nitrophenoxy)carbimidoyl azide (7). Thermolysis of 2.13 g (5.9 mmol) of azide 7 in anisole (25 mL) gave 2.26 g of a crude mixture with 13% Thermolyzate 7X. 1H-NMR (CCl4- DMSO-d6): δ ppm 2.35 (s, 3H, CH3), 3.72 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 6.70-7.60 (m, 8H, aromatics), 7.75 (d, 2H, J=9 Hz, aromatics), 8.05 (d, 2H, J=9 Hz, aromatics), 9.15 (s, H, NH), 9.74 (s, 1H, NH) with 52% N-4-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(4-nitrophenoxy)isourea (7P) and 48 % N-2-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(4-nitrophenoxy)isourea (7O).
N'-(p-toluenesulfonyl) (2,4,6-trimethylphenoxy)carbimidoyl azide (8). Thermolysis of 5.48 g (16 mmol) of azide 8 in anisole (25 mL) gave 7.38 g of crude mp. 115-120 oC with 3.5% Thermolyzate 8X. 1H-NMR (CCl4- DMSO-d6): δ ppm 2.0 (s, 6H, CH3), 2.27 (s, 3H, CH3), 2.40 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.68 (s, 2H, aromatic), 6.90-7.20 (m, 4H, aromatic), 7.25 (d, 2H, J=9Hz, aromatic), 7.72 (d, 2H, J=9Hz, aromatic), 9.50 (s, 1H, NH), 9.70 (s, 1H, NH). 1H-NMR indicated 54.5% p-N-4-(methoxy-phenyl)-N'-(4-methylphenylsulfonyl)-O-(2,4,6-trimethylphenoxy)isourea (8P) (pure 8P could not be obtained for NMR) and 45.5% N-2-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(2,4,6-trimethyl-phenoxy)isourea (8O):1H-NMR (CCl4- DMSO-d6): δ ppm 2.0 (s, 6H, CH3), 2.32 (s, 3H, CH3), 2.40 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 4.05 (s, 3H, OCH3), 6.70 (s, 2H, aromatic), 6.73-6.85 (m, 4H, aromatic), 7.40 (d, 2H, J=9 Hz, aromatic), 7.70 (d, 2H, J=9 Hz, aromatic), 9.45 (s, 1H, NH), 10.0 (s, 1H, NH).
N'-(mesitylsulfonyl)(2,4,6-trimethylphenoxy)carbimidyyl azide (9). Thermolysis of 2.0 g (5.18 mole) of azide 9 in anisole (40 mL) produced a crude mixture with 14.5% Thermolyzate 9X. 1H-NMR (CCl4- DMSO-d6) δ ppm 1.90 (s, 6H, CH3), 2.11 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.51 (s, 6H, CH3), 3.85 (s, 3H, OCH3), 4.11 (s, 3H, OCH3), 6.81 (d, 2H, J=9Hz, aromatic), 7.10 (d, 2H, J=9Hz, aromatic), 7.15 (s. 3H, aromatic), 9.65 (s, 1H, NH), 10.2 (s, 1H, NH). Crystallization from methanol gave 1.2 g (46%) of N-4-(methoxyphenyl)-N'-(4-mesitylsulfonyl)-O-(2,4,6-trimethylphenoxy)isourea (9P), mp.175-6oC. 1H-NMR (CCl4-DMSO-d6) δ ppm 1.80 (s, 6H, CH3), 2.20 (s, 3H, CH3), 2.36 (s, 3H, CH3), 2.50 (s, 6H, CH3), 4.0 (s, 3H, OCH3), 6.75 (d, 2H, J=9Hz, aromatic), 7.10 (d, 2H, J=9Hz, aromatic), 7.60 (s, 2H, aromatics), 7.70 (s, 2H, aromatics), 10.15 (s, 1H, NH). No pure N-2-(methoxyphenyl)-N'-(4-methylphenylsulfonyl)-O-(phenoxy)isourea (9O) could be isolated.
N'-Cyano(2,4,6-trimethylphenoxy)carbimidyl azide (10). Thermolysis of 0.20 g of azide 10 in anisole (7 mL) produced a crude mixture (3.5 % Thermolyzate 10X) of 69% N-4-(methoxyphenyl)-N'-(cyano)-O-(2,4,6-trimethylphenoxy)isourea (10P) and 31% N-2-(methoxyphenyl)-N'-(cyano)-O-(2,4,6-trimethyl-phenoxy)isourea (10O). 1H-NMR (CDCl3) δ ppm 2.1 (s, 6H, CH3), 2.35 (s, 3H, CH3), 3.8 (s, 3H, OCH3), 4.05 (s, 3H, OCH3), 6.85-7.40 (m, 6H, aromatics), 9.5 (s, 1H, NH). Attempted separation of the isomers was not successful.