Correlation between Phenylphenalenone Phytoalexins and Phytopathological Properties in Musa and the Role of a Dihydrophenylphenalene Triol
Abstract
:Introduction
Results and Discussion
Phytoalexin production and pathogen resistance
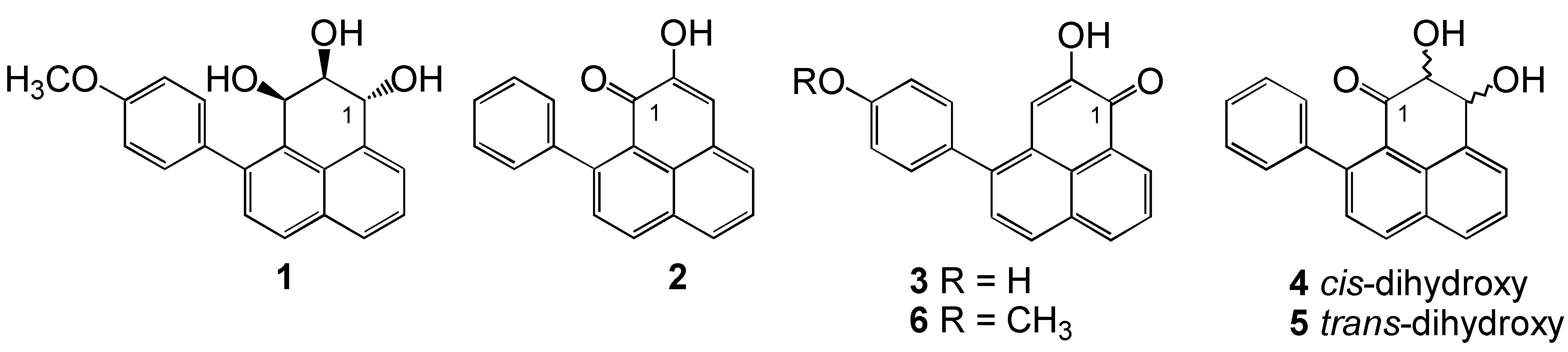
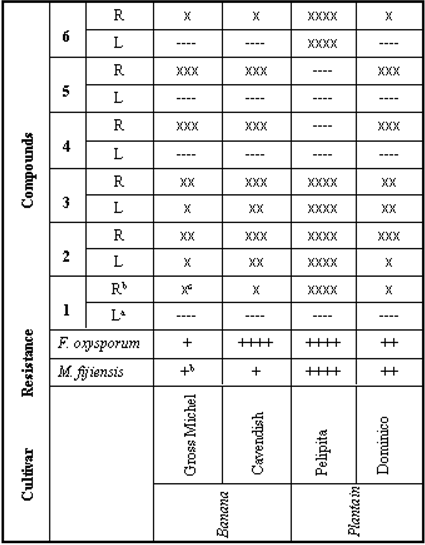 |
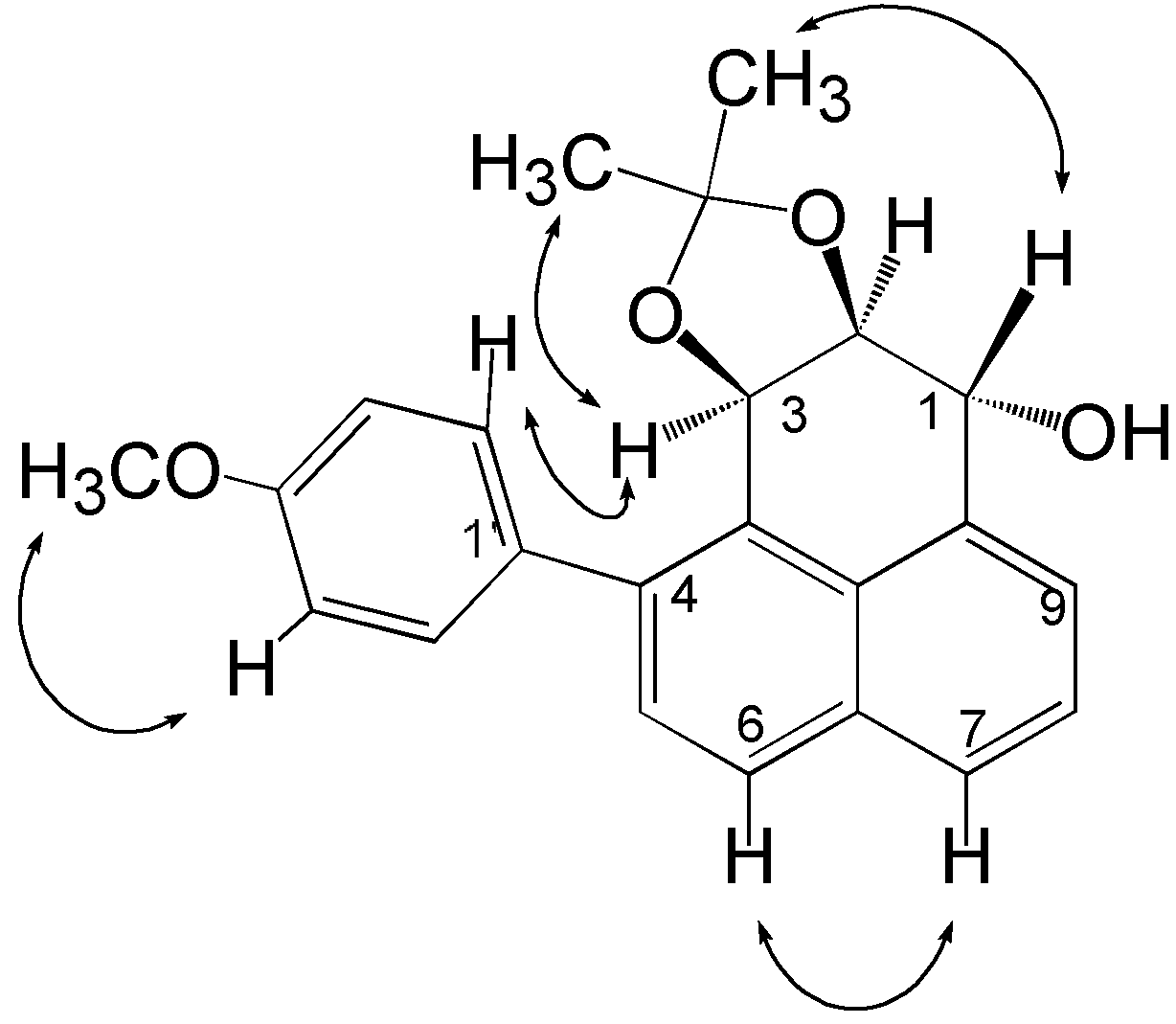
Structure elucidation and stereochemistry of compound 1
| Compound 1 | Compound 7 | |||
|---|---|---|---|---|
| δ 1H (J in Hz) | δ 13C | δ 1H (J in Hz) | δ 13C | |
| 1 | 5.28 (d, J=9.6) | 70.6 | 5.19 (d, J=8.3) | 71.6 |
| 2 | 3.83 (dd, J=9.6, 2.9) | 75.3 | 4.17 (dd, J=8.3, 4.9) | 80.1 |
| 3 | 5.02 (d, J=2.9) | 68.7 | 4.99 (d, J=4.9) | 73.5 |
| 3a | - | 130.0 | - | 129.3 |
| 4 | - | 140.9 | - | 143.0 |
| 5 | 7.48 (d, J=8.5) | 129.3 | 7.56 (d, J=8.5) | 129.8 |
| 6 | 7.90 (d, J=8.5) | 129.2 | 7.96 (d, J=8.5) | 123.2 |
| 6a | - | 133.0 | - | 132.7 |
| 7 | 7.83 (d, J=7.7) | 127.8 | 7.84 (d, J=7.6) | 129.3 |
| 8 | 7.57 (t, J =7.7) | 126.4 | 7.57 (t, J=7.6 ) | 126.4 |
| 9 | 7.83 (d, J=7.7) | 123.3 | 7.86 (d, J=7.6) | 125.2 |
| 9a | - | 136.0 | - | 134.9 |
| 9b | - | 128.0 | - | 125.3 |
| 1´ | - | 133.2 | - | 133.1 |
| 2´- 6´ | 7.53 (d, J=8.6) | 131.3 | 7.66 (d, J=8.6) | 131.9 |
| 3´- 5´ | 7.00 (d, J=8.6) | 114.1 | 7.00 (d, J=8.6) | 113.6 |
| 4´ | - | 159.6 | - | 159.4 |
| -OCH3 | 3.88 (s) | 55.8 | 3.90 (s) | 55.8 |
| CMe2 | - | - | 1.65 (s), 1.41 (s) | 29.5, 26.7 |
| CMe2 | - | - | - | 109.5 |
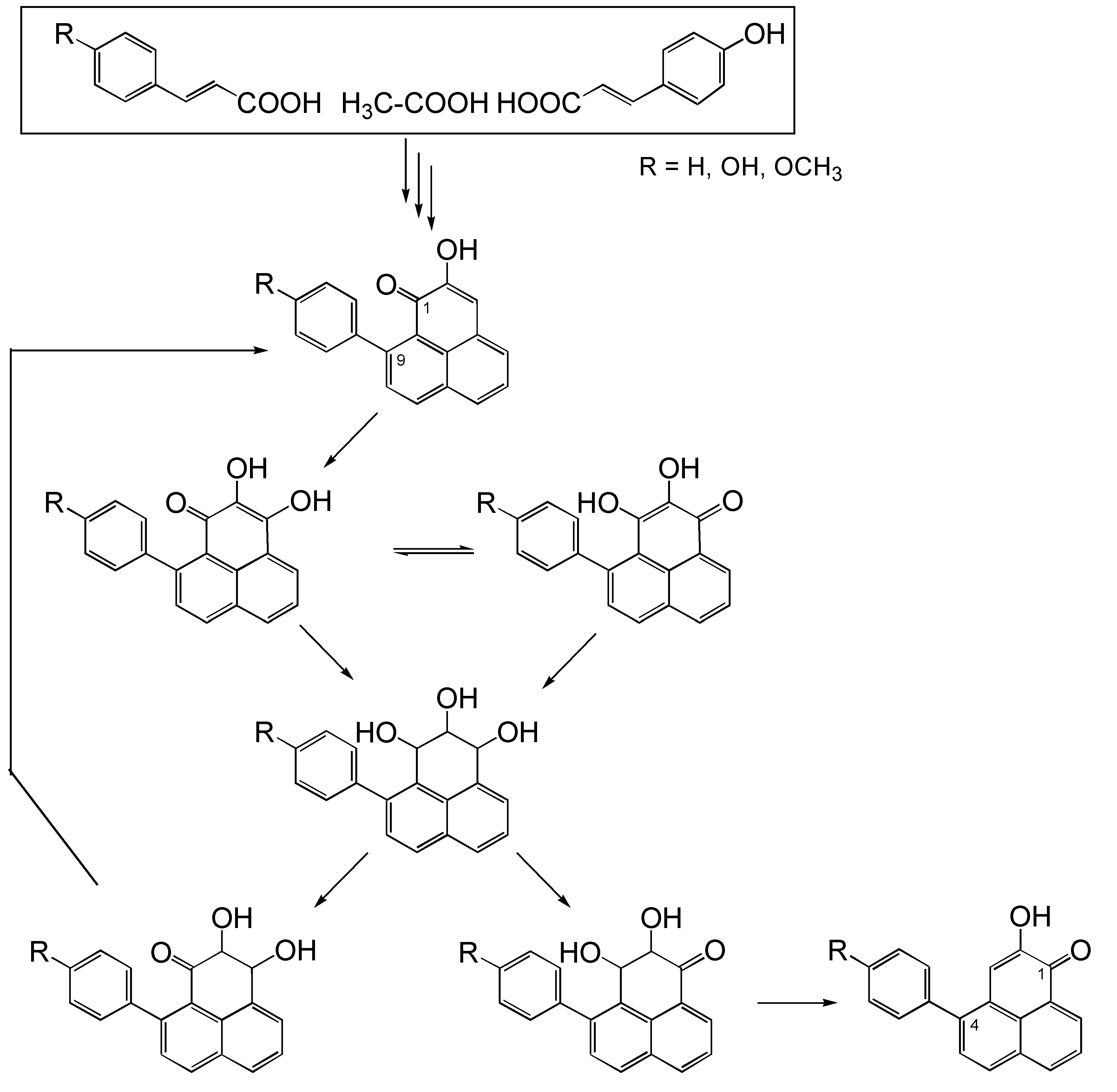
Biosynthetic pathway model
Experimental
General
Acknowledgements
References
- Grayer, R. J.; Kokubun, T. Plant-fungal interactions: the search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar]
- Luis, J. G.; Quiñones, W.; Echeverri, F.; Grillo, T. A.; Kishi, M.; García-García, F.; Torres, F.; Cardona, G. Musanolones: four 9-phenylphenalenones from rhizomes of Musa acuminata. Phytochemistry 1996, 41, 753–757. [Google Scholar] [CrossRef]
- Hölscher, D.; Schneider, B. Phenylphenalenones from Ensete ventricosum. Phytochemistry 1998, 49, 2155–2157. [Google Scholar] [CrossRef]
- Cooke, R. G.; Edwards, J. M. Naturally occurring phenalenones and related compounds. Prog. Chem. Org. Nat. Prod. 1981, 40, 153–190. [Google Scholar]
- Hölscher, D.; Schneider, B. Phenalenones from Strelitzia reginae. J. Nat. Prod. 2000, 63, 1027–1028. [Google Scholar] [CrossRef]
- Greca, M. D.; Lanzetta, R.; Molinaro, A.; Monaco, P.; Previtera, L. Phenalene metabolites from Eichhornia crassipes. Bioorg. Med. Chem. Lett. 1992, 2, 311–314. [Google Scholar] [CrossRef]
- Cooke, R. G.; Thomas, R. L. Colouring matters of Australian plants. XVIII. Constituents of Anigozanthos rufus. Aust. J. Chem. 1975, 28, 1053–1057. [Google Scholar] [CrossRef]
- Luis, J. G.; Echeverri, F.; Quiñones, W.; Brito, I.; López, M.; Torres, F.; Cardona, G.; Aguiar, Z.; Rojas, M. Irenolone and emenolone-two new types of phytoalexin from Musa paradisiaca. J. Org. Chem. 1993, 58, 4306-08. [Google Scholar] [CrossRef]
- Luis, J. G.; Quiñones, W.; Echeverri, F.; Grillo, T. A. Phenalenone–type phytoalexins from Musa acuminata. Synthesis of 4-phenylphenalenones. Tetrahedron 1994, 50, 10963–10970. [Google Scholar]
- Simmonds, N. W. Bananas, 2nd ed.; Longman: NewYork, 1982. [Google Scholar]
- Luis, J. G.; Fletcher, W. Q.; Echeverri, F.; Grillo, T. A.; Perales, A.; Gonzalez, A. Intermediates with biosynthetic implications in de novo production of phenyl-phenalenone-type phytoalexins by Musa acuminata. Revised structure of emenolone. Tetrahedron 1995, 51, 4117–4130. [Google Scholar] [CrossRef]
- Hölscher, D.; Schneider, B. A diarylheptanoid in the biosynthesis of phenylphenalenones in Anigozanthos preissii. J. Chem. Soc. Chem. Comm. 1995, 525–526. [Google Scholar]
- Hölscher, D.; Schneider, B. The biosynthetic origin of the central one-carbon unit of phenylphenalenones in Anigozanthos preissii. Nat. Prod. Lett. 1995, 7, 177–182. [Google Scholar] [CrossRef]
- Kamo, T.; Kato, N.; Hirai, N.; Tsuda, M.; Fujioka, D.; Ohigashi, H. Phenylphenalenone-type phytoalexins from unripe Buñgulan banana fruit. Biosci. Biotech. Biochem. 1998, 62, 95–101. [Google Scholar] [CrossRef]
- Kamo, T.; Hirai, N.; Tsuda, M.; Fujioka, D.; Ohigashi, H. Changes in the content and biosynthesis of phytoalexins in banana fruit. Biosci. Biotech. Biochem. 2000, 64, 2089–2098. [Google Scholar]
- Kamo, T.; Hirai, N.; Wami, K.; Fujioka, D.; Ohigashi, H. New phenylphenalenones from banana fruit. Tetrahedron 2001, 57, 7649–7656. [Google Scholar] [CrossRef]
- Luis, J. G.; Fletcher, W. Q.; Echeverri, F.; Abad, T.; Kishi, M. P.; Perales, A. New phenalenone-type phytoalexins from Musa acuminata (Colla AAA) Grand Nain. Nat. Prod. Lett. 1995, 6, 23–30. [Google Scholar] [CrossRef]
- Hoss, R.; Helbig, J.; Bochow, H. Function of host and fungal metabolites in resistance response of banana and plantain in the black sigatoka disease pathosystem Musa sp. Mycosphaerella fijiensis. J. Phytopathol. 2000, 148, 387–394. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Otálvaro, F.; Echeverri, F.; Quiñones, W.; Torres, F.; Schneider, B. Correlation between Phenylphenalenone Phytoalexins and Phytopathological Properties in Musa and the Role of a Dihydrophenylphenalene Triol. Molecules 2002, 7, 331-340. https://doi.org/10.3390/70300331
Otálvaro F, Echeverri F, Quiñones W, Torres F, Schneider B. Correlation between Phenylphenalenone Phytoalexins and Phytopathological Properties in Musa and the Role of a Dihydrophenylphenalene Triol. Molecules. 2002; 7(3):331-340. https://doi.org/10.3390/70300331
Chicago/Turabian StyleOtálvaro, Felipe, Fernando Echeverri, Winston Quiñones, Fernando Torres, and Bernd Schneider. 2002. "Correlation between Phenylphenalenone Phytoalexins and Phytopathological Properties in Musa and the Role of a Dihydrophenylphenalene Triol" Molecules 7, no. 3: 331-340. https://doi.org/10.3390/70300331





