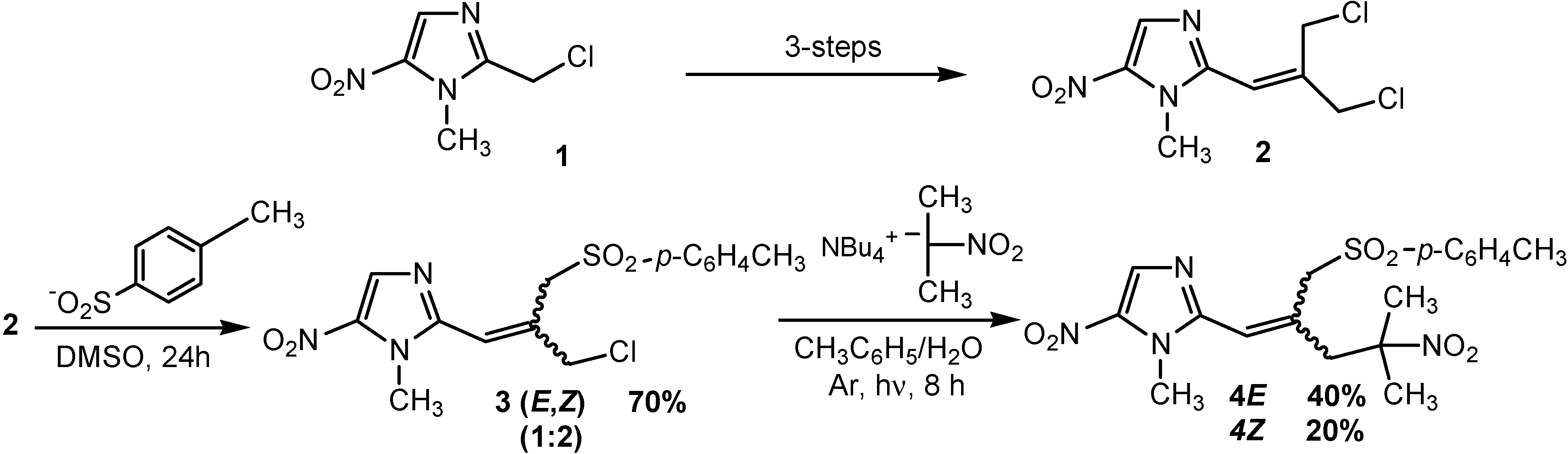
E isomer
1H-NMR (CDCl3) δ: 2.49 (s, 3H, CH3), 3.88 (s, 3H, NCH3), 4.14 (s, 2H, CH2Cl), 4.89 (s, 2H, CH2SO2), 6.31 (s, 1H, CH=C), 7.41 (d, 2H, J = 8.2 Hz, CHAr), 7.81 (d, 2H, J = 8.2 Hz, CHAr), 8.02 (s, 1H, CHImid); 13C-NMR (CDCl3) δ: 22.3 (CH3), 33.8 (NCH3), 42.8 (CH2Cl), 62.0 (CH2SO2), 121.1 (CH=C), 129.1 (2 x CHAr), 130.8 (2 x CHAr), 133.8 (CHImid), 136.8 (Cq), 139.0 (Cq), 146.2 (Cq), 146.6 (Cq).
Z isomer
1H-NMR (CDCl3) δ: 2.37 (s, 3H, CH3), 3.71 (s, 3H, NCH3), 4.55 (s, 2H, CH2Cl), 5.05 (s, 2H, CH2SO2), 6.54 (s, 1H, CH=C), 7.21 (d, 2H, J = 8.2 Hz, CHAr), 7.63 (d, 2H, J = 8.2 Hz, CHAr), 7.88 (s, 1H, CHImid). 13C-NMR (CDCl3) δ: 22.1 (CH3), 33.5 (NCH3), 48.7 (CH2Cl), 55.8 (CH2SO2), 119.2 (CH=C), 129.1 (2 x CHAr), 129.7 (2 x CHAr), 133.2 (CHImid), 136.2 (Cq), 137.7 (Cq), 145.7 (Cq), 146.8 (Cq).
SRN1 reaction of chloride 3 and 2-nitropropane. Under nitrogen atmosphere, a solution of tetrabutylammonium hydroxide (1.6M/water, 3.6 mL, 5.4 mmol) was treated with 2-nitropropane (0.48 g, 5.4 mmol) for 1 h. A solution of chloride 3 (0.5 g, 1.35 mmol) in toluene (10 mL) was added and the mixture was irradiated with a 300W sun lamp for 8 h. The organic layer was separated and the aqueous layer was extracted with dichloromethane (3 x 10 mL). The combined organic layers were washed twice with water (2 x 30 mL), dried over MgSO4 and removed under reduced pressure. Purification by chromatography on silica gel eluting with chloroform-ethyl acetate (95/5) and recrystallization from ethanol gave the 3-(1-methyl-5-nitro-1H-imidazol-2-yl)-2-(2-methyl-2-nitropropyl)prop-2-ene-1-sulfinic acid p-tolyl ester (4E) (0.23 g, 40% yield) and (4Z) (0.12 g, 20% yield).
3-(1-methyl-5-nitro-1H-imidazol-2-yl)-2-(2-methyl-2-nitropropyl)prop-2-ene-1-sulfinic acid p-tolyl ester (4E): Yellow solid, mp 115 °C (ethanol). 1H-NMR (CDCl3) δ: 1.67 (s, 6H, (CH3)2CNO2), 2.32 (s, 3H, CH3), 3.17 (s, 2H, CH2C(CH3)2NO2), 3.57 (s, 3H, NCH3), 4.90 (s, 2H, CH2SO2), 6.03 (s, 1H, CH=C), 7.16 (d, 2H, J = 8.2 Hz, CHAr), 7.55 (d, 2H, J = 8.2 Hz, CHAr), 7.82 (s, 1H, CHImid). 13C-NMR (CDCl3) δ: 22.1 (CH3), 26.8 (2 x CH3), 33.4 (NCH3), 47.7 (CH2C(CH3)2NO2), 58.9 (CH2SO2), 89.4 (C(CH3)2NO2), 121.1 (CH=C), 129.2 (2 x CHAr), 129.7 (2 x CHAr), 133.1 (CHImid), 135.9 (Cq), 136.5 (Cq), 145.6 (Cq), 147.0 (Cq). Anal. Calcd for C18H22N4O6S : C, 51.18; H, 5.25; N, 13.26; S, 7.59. Found : C, 51.15; H, 5.21; N, 13.30; S, 7.52.
3-(1-methyl-5-nitro-1H-imidazol-2-yl)-2-(2-methyl-2-nitropropyl)prop-2-ene-1-sulfinic acid p-tolyl ester (4Z): Yellow solid, mp 118 °C (ethanol). 1H-NMR (CDCl3) δ: 1.61 (s, 6H, (CH3)2CNO2), 2.48 (s, 3H, CH3), 3.57 (s, 2H, CH2C(CH3)2NO2), 3.80 (s, 2H, CH2SO2), 3.95 (s, 3H, NCH3), 6.53 (s, 1H, CH=C), 7.40 (d, 2H, J = 8.2 Hz, CHAr), 7.78 (d, 2H, J = 8.2 Hz, CHAr), 8.03 (s, 1H, CHImid). 13C-NMR (CDCl3) δ: 22.3 (CH3), 26.6 (2 x CH3), 33.7 (NCH3), 41.7 (CH2C(CH3)2NO2), 63.5 (CH2SO2), 89.0 (C(CH3)2NO2), 122.9 (CH=C), 129.0 (2 x CHAr), 130.7 (2 x CHAr), 133.5 (CHImid), 136.2 (Cq), 136.4 (Cq), 139.4 (Cq), 146.1 (Cq), 147.4 (Cq). Anal. Calcd for C18H22N4O6S : C, 51.18; H, 5.25; N, 13.26; S, 7.59. Found : C, 51.15; H, 5.30; N, 13.20; S, 7.80.