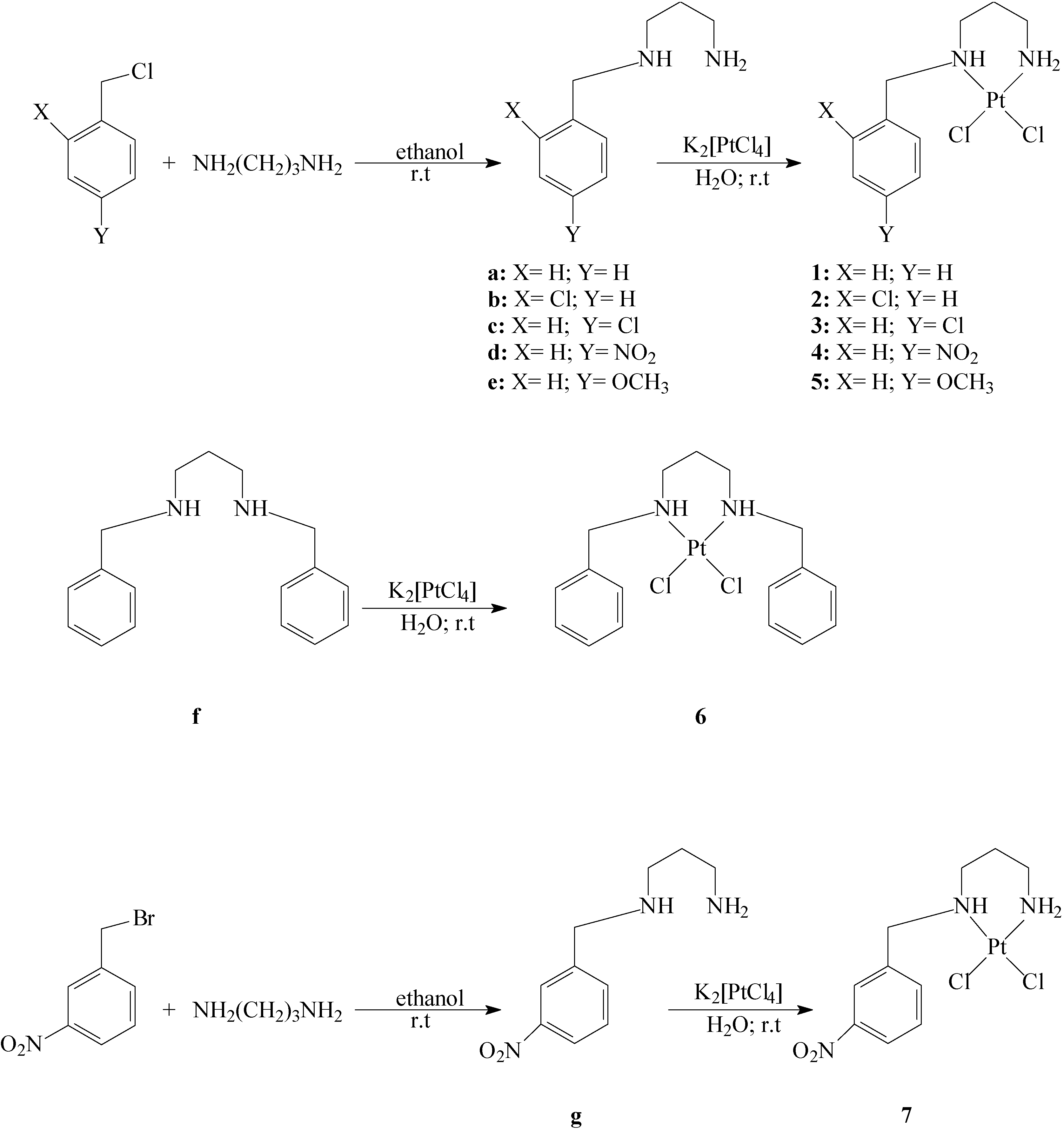General
IR spectra were obtained on a Bomem FT IR MB-102 spectrometer in KBr pellets. 1H-NMR (200 and 400 MHz), 13C-NMR (50 and 100 MHz) and 195Pt-NMR (86 MHz) spectra were recorded on Bruker Advance DRX 200 and DRX 400 spectrometers at the Federal University of Minas Gerais, Brazil. Elemental analyses were done at the State University of São Paulo, Brazil. All chemicals were reagent grade and were used without further purification.
Synthesis of ligands: N-Benzylpropane-1,3-diamine (a), N-(2-chlorobenzyl)propane-1,3-diamine (b), N-(4-chlorobenzyl)propane-1,3-diamine (c), N-(4-nitrobenzyl)propane-1,3-diamine (d), N-(4-methoxy-benzyl)propane-1,3-diamine (e), N,N’-dibenzylpropane-1,3-diamine (f) and N-(3-nitrobenzyl)-propane-1,3-diamine (g).
To 1,3-propanediamine (3.34 mL; 40 mmols) in ethanol (20 mL), the corresponding benzyl halide (20 mmol) was slowly added during 8 h. The reaction mixture was stirred for 48 h at room temperature, after which time, the complete consumption of the starting material was evidenced by TLC (eluent: 8:2 hexane/ethyl acetate). A saturated sodium hydroxide (30 mmol) solution in ethanol was then slowly added. The solvent was evaporated under reduced pressure, and the residue purified on silica gel 60 G (0.2-0.5 mm), using 9:1 dichloromethane/methanol as eluent. Yields: (a) 2.65 g (81%); (b) 2.89 g (73%); (c) 2.81 g (71%); (d) 3.26 g (78%); (e) 2.67 g (69%); (g) 4.9 g (69 %) for compound g. The N,N’-dibenzylated compound (f) was also isolated in 10% yield in the preparation of compound a.
Compound a: IR νmax. KBr (cm-1): 3238, 3025, 2982, 2907, 2763, 1599, 1438, 1176, 975, 745; 1H‑NMR (200 MHz; TFA-d1) δ: 2.12 (m, 2H, CH2); 3.09 (m, 4H, CH2N); 4.06 (s, 2H, CH2Ph); 7.15 (m, 5H, Ph); 13C-NMR (50 MHz, TFA-d1) δ: 26.17; 40.25; 47.06; 55.60 (CH2);108.50; 114.14; 120.77; 125.41 (Ph); MS (m/z, %): 165 (100.00); 148 (1.73); 134 (1.15); 120 (1.00).
Compound b: IR νmax. KBr (cm-1): 3285, 3063, 2931, 1573, 1470, 1117, 1050, 751; 1H-NMR (200 MHz; pyridine-d5) δ: 1,88 (m, 2H, CH2); 2.77 (t, 2H, CH2NH2); 2.99 (t, 2H, CH2NH); 4,39 (s, 2H, CH2Ph); 7,16 (m, 2H, H4, H5); 7,28; 7,59 (2d, 2H, H3, H6, J3-4= 7,6 Hz, J6-5= 7,3 Hz); 13C-NMR (50 MHz; pyridine-d5) δ: 32.31; 40.31; 47.57; 51.24 (CH2); 127.64; 128.48; 129.60; 130.36; 133.71; 138.87 (Ph); MS (m/z, %): 199 (100.00); 182 (6.36); 125 (1.00).
Compound c: IR νmáx. KBr (cm-1): 3439, 3050, 2934, 2788, 1578, 1498, 1438, 1091, 1018, 805; 1H‑NMR (200 MHz; DMSO-d6) δ: 2.07 (m, 2H, CH2); 2.86 (m, 4H, CH2N); 4.07 (s, 2H, CH2Ph); 7.48; 7.61 (2d, 4H, Ph); 13C-NMR (50 MHz; DMSO-d6) δ: 23.91; 36.29; 43.87; 49.46 (CH2); 128.48; 131.89; 132.05; 133.37 (Ph); MS (m/z, %): 199 (100.00); 182 (12.71); 125 (76.88).
Compound d: IR νmax. KBr (cm-1): 3380, 3090, 2955, 1603, 1519, 1351, 1107, 850, 740; 1H-NMR (200 MHz; D2O) δ: 2.00 (m, 2H, CH2); 3.00 (m, 4H, CH2N); 4.21 (s, 2H, CH2Ph); 7.57; 8.19 (2d, 4H, Ph); 13C-NMR (50 MHz; D2O) δ: 24.20; 36.58; 44.58; 50.47 (CH2); 124.11; 130.59; 139.59; 147.86 (Ph); MS (m/z, %): 210 (86.12); 193 (52.60); 179 (10.40); 165 (17.34); 136(34.68).
Compound e: IR νmax. KBr (cm-1): 3244, 3007, 2934,1614, 1542, 1513, 1479, 1249, 1180, 1030, 811; 1H-NMR (200 MHz; D2O) δ: 1.45 (m, 2H, CH2); 2.39 (m, 2H, CH2NH2); 2.55 (m, 2H, CH2NH); 3.48 (s, 2H, CH2Ph); 3.59 (s, 3H, OCH3); 6.73; 7.06 (2d, 4H, Ph); 13C-NMR (50 MHz, D2O) δ: 28.74; 38.18; 45.07; 51.52 (CH2); 55.24 (OCH3); 113.96; 130.10; 130.61; 158.8 (Ph); MS (m/z, %): 195 (94.79); 178 (0.58); 164 (1.73); 150 (1.00); 136 (1.00); 121 (100.00).
Compound f: IR νmax. KBr (cm-1): 3159, 3037, 2981, 1590, 1444, 1280, 1213, 1073, 944, 913, 860, 775, 738, 690, 607; 1H-NMR (200 MHz; TFA d1) δ: 2.55 (m, 2H, CH2); 3.36 (m, 4 H, CH2NH); 4.30 (sl, 4 H, CH2Ph); 7.41 (s, 10 H, Ph); 7.81 (sl, 2H, NH); 13C-NMR (50 MHz, TFA d1) δ: 25.73; 47.34; 55.61 (CH2); 128.86; 129.78; 131.05; 133.11 (Ph); MS (m/z, %): 255 (100.00); 148 (1.00); 134 (1.00).
Compound g: IR νmax. KBr (cm-1): 3399, 3080, 2944, 1598, 1537, 1462, 1354, 1102, 939, 736; 1H‑NMR (200 MHz; DMSO d6) δ: 2.01 (m, 2H, CH2); 2.96 (t, 2H, CH2NH2); 3.12 (t, 2H, CH2NH); 4.24 (s, 2H, CH2Ph); 7.69 (m, 1H, H5); 8.06; 8.23 (2d, 2H, H4, H6); 8.48 (s, 1H, H2); 13C-NMR (50 MHz; DMSO d6) δ: 23.93; 36.33; 44.14; 49.33 (CH2); 123.74; 125.14; 130.21; 134.75; 137.13; 147.73 (Ph); MS (m/z, %): 210 (100.00); 193 (43.93); 179 (1.15); 165 (7.51); 136 (26.01).
Synthesis of complexes: (N-Benzylpropane-1,3-diamine)dichloroplatinum(II) (1), [N-(2-chlorobenzyl)-propane-1,3-diamine]dichloroplatinum(II) (2); [N-(4-chlorobenzyl)propane-1,3-diamine]dichloro-platinum(II) (3); [N-(4-nitrobenzyl)propane-1,3-diamine]dichloroplatinum(II) (4); [N-(4-methoxy-benzyl)propane-1,3-diamine]dichloroplatinum(II) (5); (N,N’-dibenzylpropane-1,3-diamine)dichloro-platinum (II) (6) and [N-(3-nitrobenzyl)propane-1,3-diamine]dichloroplatinum(II) (7):
To K2[PtCl4] (0.415 g; 1 mmol) in water (5 mL), the appropriate ligand (1 mmol) dissolved in water (5 mL) was slowly added. After stirring for 48 h at room temperature, the product was isolated by filtration and dried. Yields: 0.22 g (52 %) for compound 1; 0.31 g (66 %) for compound 2; 0.17 g (37 %) for compound 3; 0.14 g (67 %) for compound 4; 0.17 g (38 %) for compound 5; 0.17 g (34 %) for compound 6 and 0.25 g (53 %) for compound 7.
Compound 1: IR νmax. KBr (cm-1): 3207, 3135, 3031, 2946, 2879, 1598, 1456, 1048, 748, 701, 517, 328; 1H-NMR (400 MHz; DMSO-d6) δ: 1.63 (m, 2H, CH2); 2.50; 2.63 (2m, 4H, CH2NH2, CH2NH); 4.00; 4.41 (2dd, 2H, H7, H7’, J7-7’= 13 Hz, J7-NH= 3 Hz, J7’-NH= 4 Hz); 5.12; 6.00 (2sl, 3H, NH2, NH); 7.58 (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6) δ: 23.45; 42.41; 46.74; 55.78 (CH2); 128.24; 128.62; 129.50; 130.55; 131.20 (Ph); 195Pt-NMR (86 MHz, DMSO-d6) δ: - 2254; Anal. Calcd. for C10H16N2Cl2Pt·2HCl: C: 23.85; H: 3.57; N: 5.56; found: C: 23.35; H: 3.43; N: 5.56.
Compound 2: IR νmax. KBr (cm-1): 3238, 3211, 3134, 3067, 2993, 2940, 1601, 1447, 1180, 1054, 1035, 860, 748, 551, 435, 315; 1H-NMR (200 MHz; DMSO-d6) δ: 1.96 (m, 2H, CH2); 2.37 (m, 2H, CH2NH2); 2.79 (sl ,2H, CH2NH); 4.09; 4.84 (2m, 2H, H7, H7’); 5.78; 6.63 (2m, 3H, NH2, NH); 7.42; 7.44 (2d, 2H, H3, H6); 7.51; 8.22 (2t, 2H, H4, H5); 13C-NMR (50 MHz, CDCl3) δ: 22.99; 41.81; 51.20; 54.21 (CH2); 127.28; 129.57; 131.03; 132.16; 133.86; 135.19 (Ph); 195Pt-NMR (86 MHz, DMSO-d6) δ: - 2250; Anal. Calcd. for C10H15Cl3N2Pt: C: 25.85; H: 3.25; N: 6.03; found: C: 26.26; H: 3.48; N: 5.71.
Compound 3: IR νmax. KBr (cm-1): 3244, 3207, 3135, 3030, 2934, 2879, 1596, 1575, 1492, 1180, 1092, 1013, 848, 815, 527, 493, 320; 1H-NMR (400 MHz; DMSO-d6) δ: 1.75 (m, 2H, CH2); 2.49; 2.70 (2m, 4H, CH2NH2, CH2NH); 3.92; 4.33 (2dd, 2H, H7, H7’, J7-7’= 12 Hz, J7-NH= 4 Hz, J7’-NH= 4 Hz); 5.20; 6.16 (2sl, 3H, NH2, NH); 7.44; 7.52 (2d, 4H, Ph); 13C-NMR (100 MHz, DMSO-d6) δ: 23.10; 42.39; 47.70; 55.07 (CH2); 128.13; 128.42; 131.89; 132.03 (Ph); 195Pt-NMR (86 MHz, DMSO-d6) δ: - 2248; Anal. Calcd. for C10H15N2Cl3Pt: C: 25.85; H: 3.25; N: 6.03; found: C: 26.37; H: 3.27; N: 5.91.
Compound 4: IR νmax. KBr (cm-1): 3258, 3228, 3111, 2951, 2935, 1609, 1516, 1459, 1350, 1190, 1109, 848, 706, 530, 327; 1H-NMR (400 MHz; DMSO- d6) δ: 1.88 (m, 2H, CH2); 2.75 (sl, 2H, CH2NH2); 2.85 (t, 2H, CH2NH); 4.00; 4.46 (2m, 2H, H7, H7’); 5.17; 6.37 (2sl, 3H, NH2, NH); 8.04; 8.28 (2d, 4H, Ph); 13C-NMR (100 MHz, DMSO-d6) δ: 23.17; 42.53; 49.05; 55.22 (CH2); 123.10; 131.69; 132.55; 147.03 (Ph); 195Pt-NMR (86 MHz, DMSO-d6) δ: - 2236; Anal. Calcd. for C10H15N3Cl2O2Pt: C: 25.27; H: 3.18; N: 8.84; found: C: 25.05; H: 3.38; N: 8.56.
Compound 5: IR ν KBr (cm-1): 3245, 3216, 3168, 3144, 3004, 2938, 1611, 1513, 1460, 1253, 1177, 1030, 817, 554, 329; 1H-NMR (400 MHz; DMSO-d6) δ: 1.49 (m, 2H, CH2); 2.40; 2.49 (2m, 4H, CH2NH2, CH2NH); 3.72 (s, 3H, OCH3); 3.92; 4.33 (2dd, 2H, H7’, H7’); 5.12; 5.85 (2sl, 3H, NH2, NH); 6.88; 7.32 (2d, 4H, Ph); 13C-NMR (100 MHz, CDCl3) δ: 23.06; 42.43; 47.05; 55.14 (CH2); 55.22 (OCH3);113.58; 131.07; 131.87; 132.45; 158.86 (Ph); 195Pt-NMR (86 MHz, DMSO-d6) δ: - 2256; Anal. Calcd. for C11H18N2Cl2OPt: C: 28.71; H: 3.94; N: 6.09; found: C: 28.77; H: 4.03; N: 5.93.
Compound 6: IR ν KBr (cm-1): 3159, 3012, 2980, 1576, 1498, 1453, 1433, 1211, 994, 748, 697, 523, 325, 315; 1H-NMR (400 MHz; DMSO-d6) δ: 2.08 (m, 2H, CH2); 3.03 (sl, 4H, CH2NH); 4.14 (sl, 4H, CH2Ph); 7.44; 7.56 (2d, 10H, Ph); Anal. Calcd. for C17H22N2Cl2Pt·4H2O: C: 34.46; H: 5.10; N: 4.72; found: C: 34.60; H: 4.67; N: 4.84.
Compound 7: IR ν KBr (cm-1): 3229, 3208, 3127, 3049, 2953, 1596, 1529, 1450, 1352, 1140, 1048, 813, 736, 702, 472, 324; 1H-NMR (400 MHz; DMSO-d6) δ: 1.71 (m, 2H, CH2); 2.64; 2.66 (2t, 4H, CH2NH2, CH2NH); 3.94; 4.44 (2sl, 2H, H7, H7’); 5.15; 6.42 (2sl, 3H, NH2, NH); 7.68 (sl ,1H, H5); 8.08; 8.22 (2d, 2H, H4, H6, J4-5= J5-6= 6.8 Hz); 8.51 (s, 1H, H2); 13C-NMR (100 MHz, DMSO-d6) δ: 23.10; 42.52; 49.19; 58.13 (CH2); 122.74; 125.24; 125.26; 129.46; 137.25; 147.38 (Ph); 195Pt-NMR (86 MHz, DMSO-d6) δ: - 2241; Anal. Calcd. for C10H15N3Cl2O2Pt ·H2O: C: 24.34; H: 3.44; found: C: 24.46; H: 2.94.