Preparation and Characterization of Double Metal Cyanide Complex Catalysts
Abstract
:Introduction
Results and Discussion
1. Effect of the additional complex ligand on catalysts
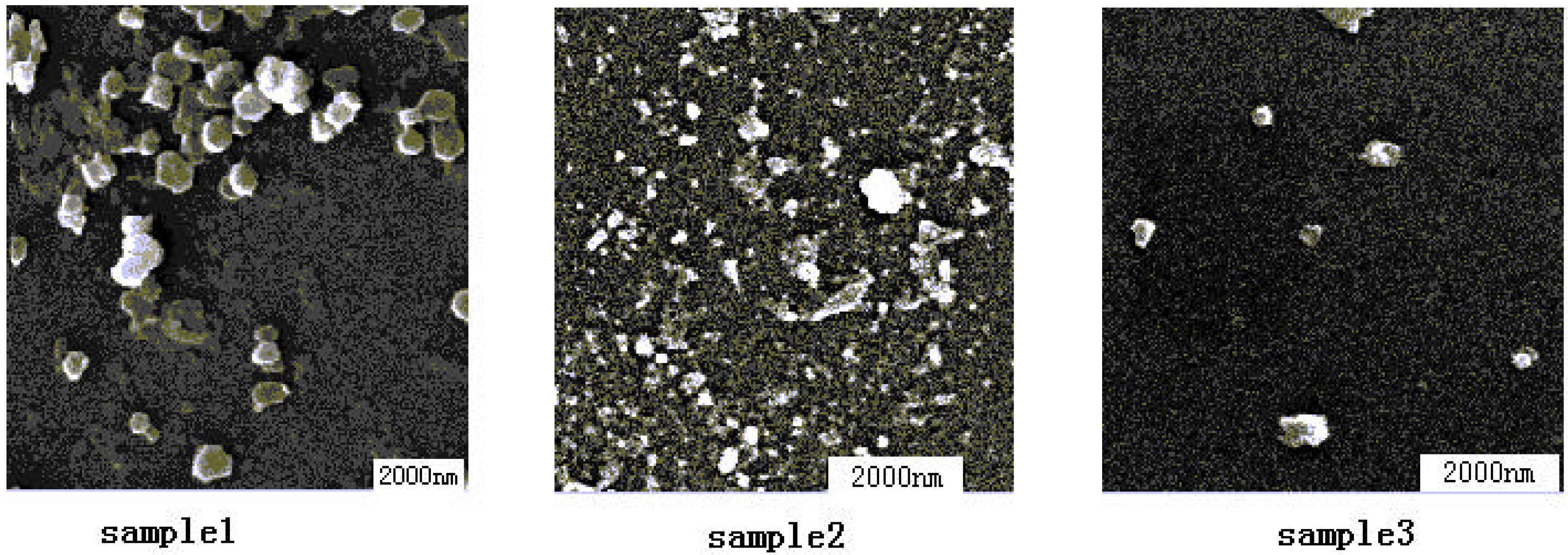

| Sample | Mean grainsize(nm) | Mean shape | |
|---|---|---|---|
| FD-0 | FD-90 | ||
| 1 | 224 | 228 | 1.75 |
| 2 | 81 | 76 | 1.08 |
| 3 | 224 | 162 | 1.28 |
| 4 | 131 | 98 | 1.41 |
| 5 | 317 | 260 | 1.87 |
| Sample | C (%) | N (%) | O (%) | Cl (%) | Ca (%) | Co (%) | Zn (%) |
|---|---|---|---|---|---|---|---|
| 1 | 43.75 | 22.42 | 15.64 | 1.51 | 0.54 | 3.65 | 12.15 |
| 2 | 25.96 | 18.67 | 27.30 | 1.31 | 1.39 | 6.69 | 18.69 |
| 3 | 30.41 | 25.00 | 13.53 | 1.61 | 0.15 | 7.50 | 21.36 |

2. The influence of the preparation method on DMC catalysts
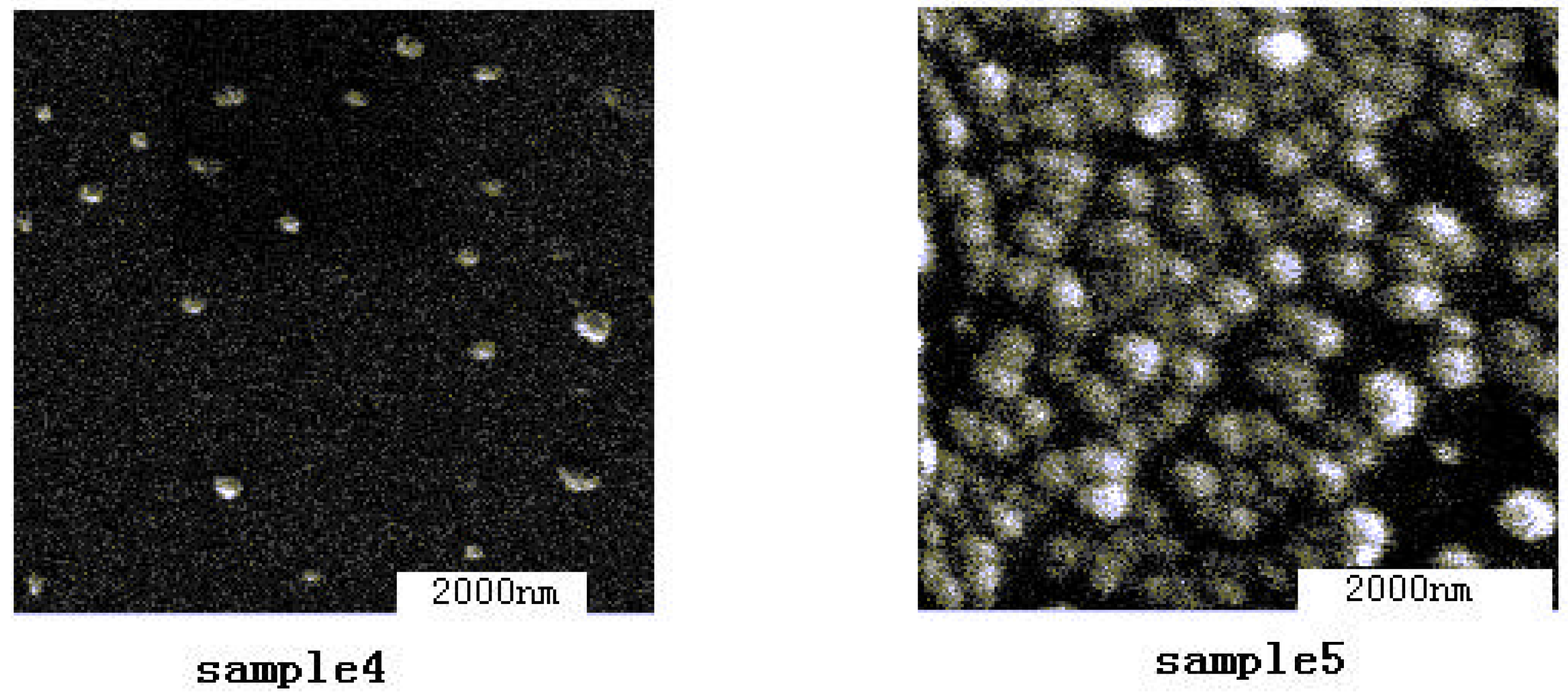
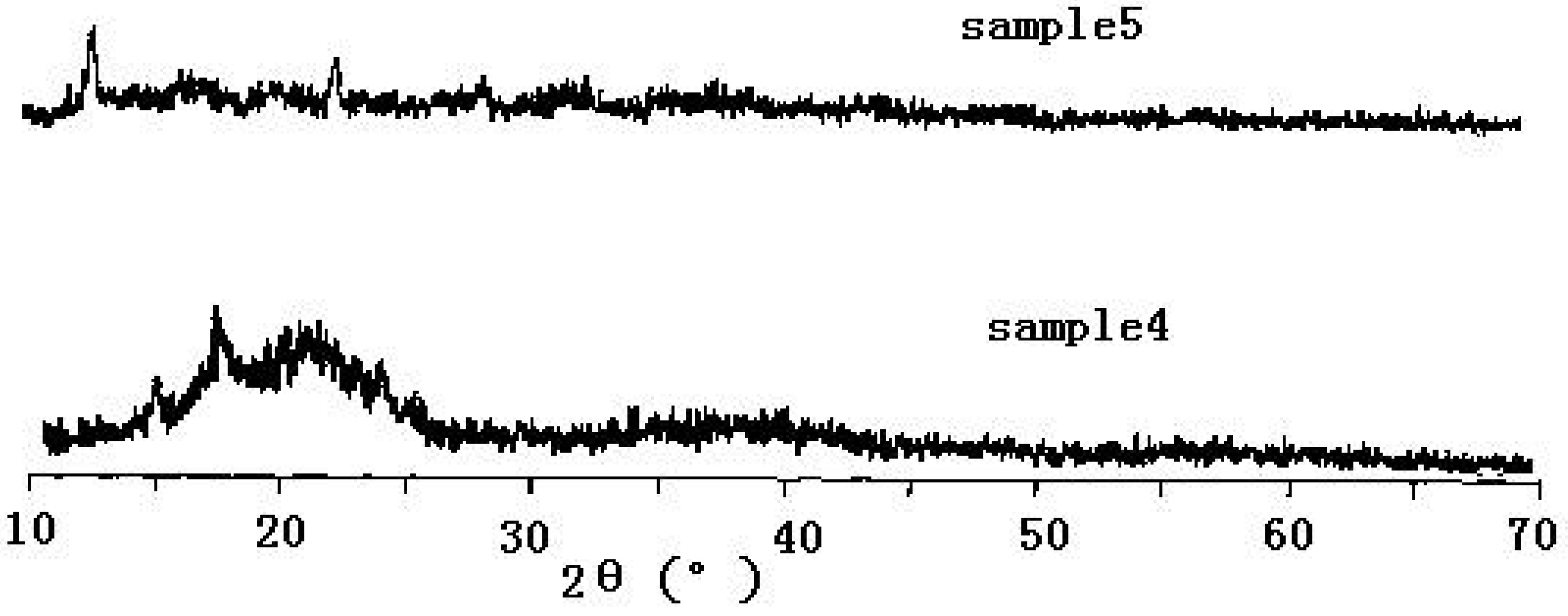
3. Catalytic properties
| Sample | Additional complex ligand | Preparation method | Catalyst concentration mg(Cat)/kg(PO) | Reaction time ( h ) | Yield kg/g |
|---|---|---|---|---|---|
| 1 | PEG-1000 | method 1 | 100 | 5.5 | 11.2 |
| 2 | β –clodextrin | method 1 | 100 | 5.0 | 12.6 |
| 3 | Tween-60 | method 1 | 100 | 5.0 | 10.8 |
| 4 | PEG-1000 | method 2 | 50 | 3.5 | 15.2 |
| 5 | Tween-60 | method 2 | 50 | 4.0 | 14.6 |
Conclusions
Experimental
General
Preparation
Characterization
Catalytic properties
References
- Robert, J.H. Polyethers and Method for Making the Same. US Patent 3,829,505, 1974. [Google Scholar]
- Robert, J.H.; Livigni, R.A. Advances in chemistry series-polymerization. Kinet Technol. 1973, 128, 208–213. [Google Scholar]
- Liu, X.H.; Kang, M.Q.; Wang, X.K. Preparation and characterization of double methyl cyanide complex catalysts. Chin. J. Mol. Catalysts 2000, 14(4), 247–250. [Google Scholar]
- Huang, Yijun; Qi, Guorong; Feng, Linxian. Preparation, characterization and catalytic performance of double methyl cyanide complex. Chin. J. Catalysis 2000, 23(2), 113–117. [Google Scholar]
- Hofmann, J.; Ooms, P.; Gupta, P.; et al. Double metal cyanide catalysts for preparing poly-etherpolyols. US Patent 6,291,388, 2001. [Google Scholar]
- Ooms, P.; Hofmann, J.; Gupta, P.; et al. Double metal cyanide catalysts for preparing poly-etherpolyols. US Patent 6,204,357, 2001. [Google Scholar]
- Combs, G. Double metal cyanide complex catalysts modified with GroupIA compounds. US Patent 5,952,261, 1999. [Google Scholar]
- Le-Khac, B.; Bowman, P.T.; Hinney, H.R. Highly double metal cyanide complex catalysts. US Patent 5,712,216, 1998. [Google Scholar]
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Liu, H.; Wang, X.; Gu, Y.; Guo, W. Preparation and Characterization of Double Metal Cyanide Complex Catalysts. Molecules 2003, 8, 67-73. https://doi.org/10.3390/80100067
Liu H, Wang X, Gu Y, Guo W. Preparation and Characterization of Double Metal Cyanide Complex Catalysts. Molecules. 2003; 8(1):67-73. https://doi.org/10.3390/80100067
Chicago/Turabian StyleLiu, Hanxia, Xikui Wang, Yao Gu, and Weilin Guo. 2003. "Preparation and Characterization of Double Metal Cyanide Complex Catalysts" Molecules 8, no. 1: 67-73. https://doi.org/10.3390/80100067




