Novel 4-Aroyl-3-alkoxy-2(5H)-furanones as Precursors for the Preparation of Furo[3,4-b][1,4]-diazepine Ring System
Abstract
:Introduction
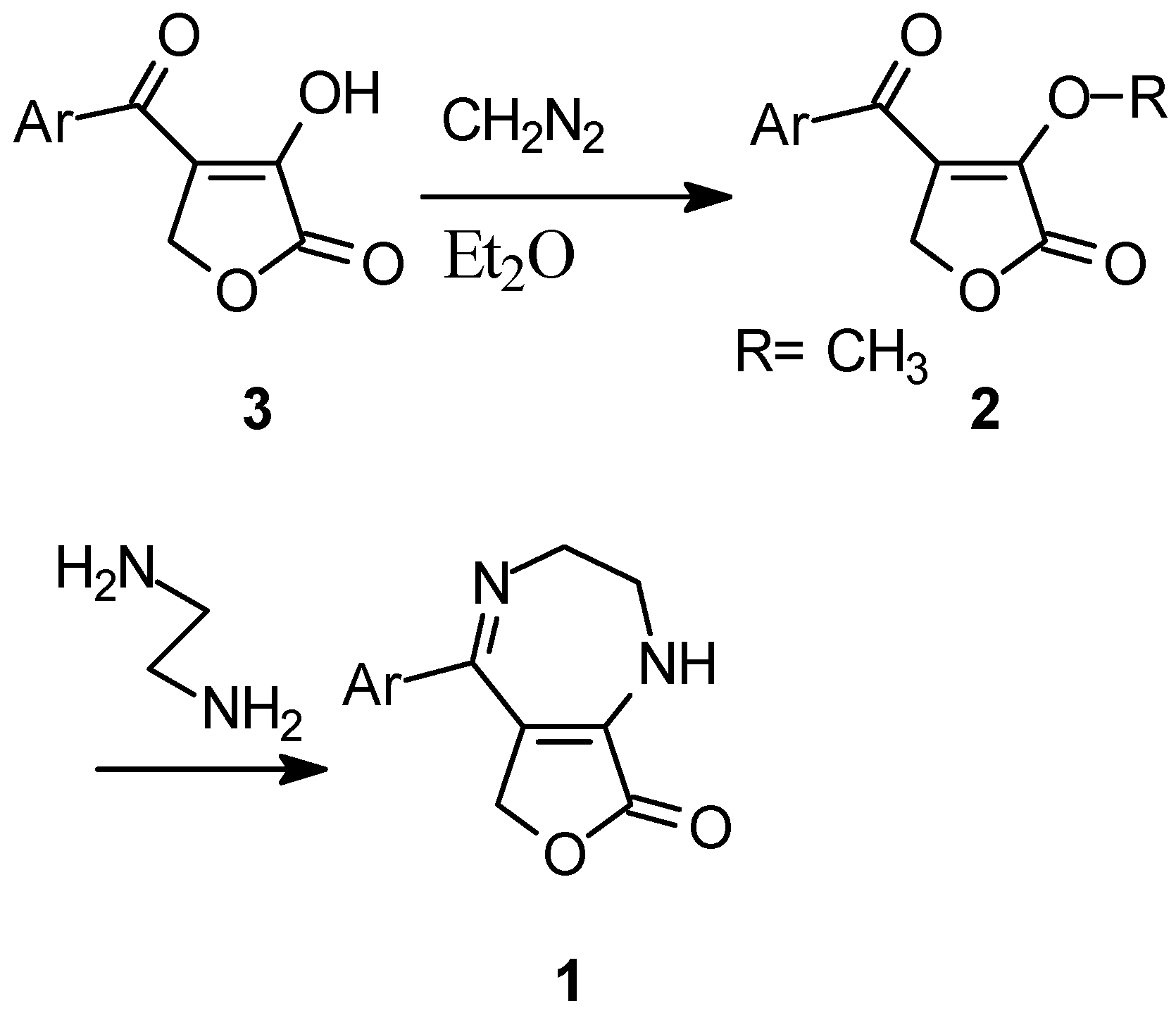
Results and Discussion
| Entry | Ar | R | Yield (%) | Mp (°C) |
|---|---|---|---|---|
| 2a | C6H5 | CH3CH2- | 42 | Oil |
| 2b | C6H5 | CH2=CHCH2- | 51 | 52 |
| 2c | o-Cl C6H4 | CH3CH2- | 35 | 48 |
| 2d | o-Cl C6H4 | (CH3)2CHCH2- | 41 | Oil |
| 2e | m-CNC6H4 | CH3- | 47 | 116 |
| 2f | p-CH3C6H4 | CH3- | 31 | Oil |
| 2g | p-CH3C6H4 | CH3CH2- | 28 | Oil |
| 2h | p-CH3C6H4 | CH3(CH2)2CH2- | 29 | 42 |
| 2i | p-CH3C6H4 | CH3(CH2)5CH2- | 31 | Oil |
| 2j | p-CH3C6H4 | C6H5CH2- | 42 | 84 |
| 2k | p-CH3C6H4 | CH2=CHCH2- | 48 | 70 |
| 2l | p-CH3C6H4 | (CH3)2CHCH2- | 17 | 55 |
| 2m | p-CH3C6H4 | CH3CH2O2CH2- | 53 | 84 |
| 2n | p-CH3C6H4 | (CH3)2CH- | 23 | 73 |
| 2o | p-CH3C6H4 | (CH2)4CH- | 25 | 80 |
| 2p | p-NO2C6H4 | CH3- | 30 | 85 |
| 2q | 5-CH3-2-thienyl | CH3- | 33 | Oil |
| 4 | p-CH3C6H4 | o-C6H4(CH2-)2 | 24 | 98 |

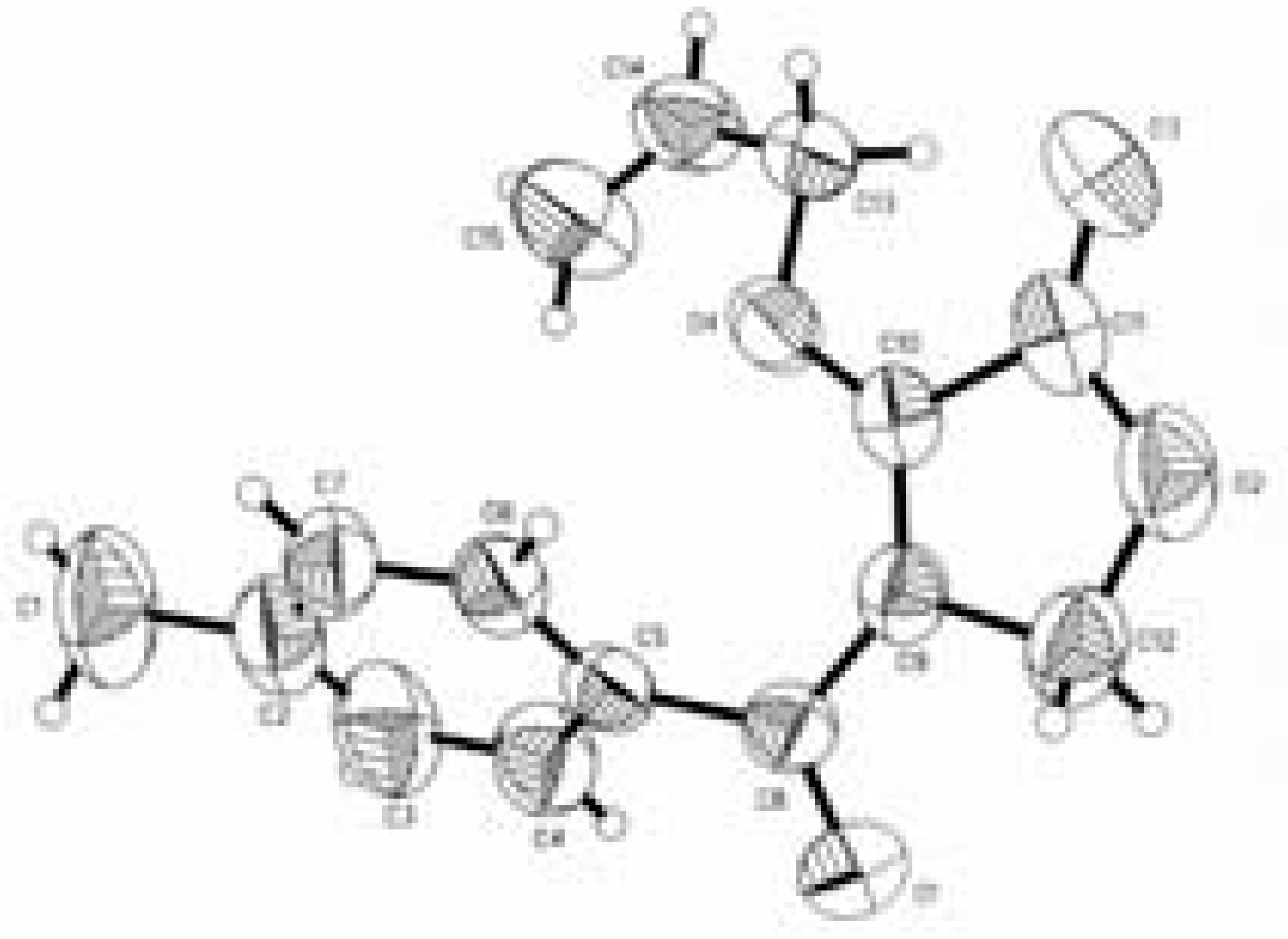
Conclusions
Experimental
General
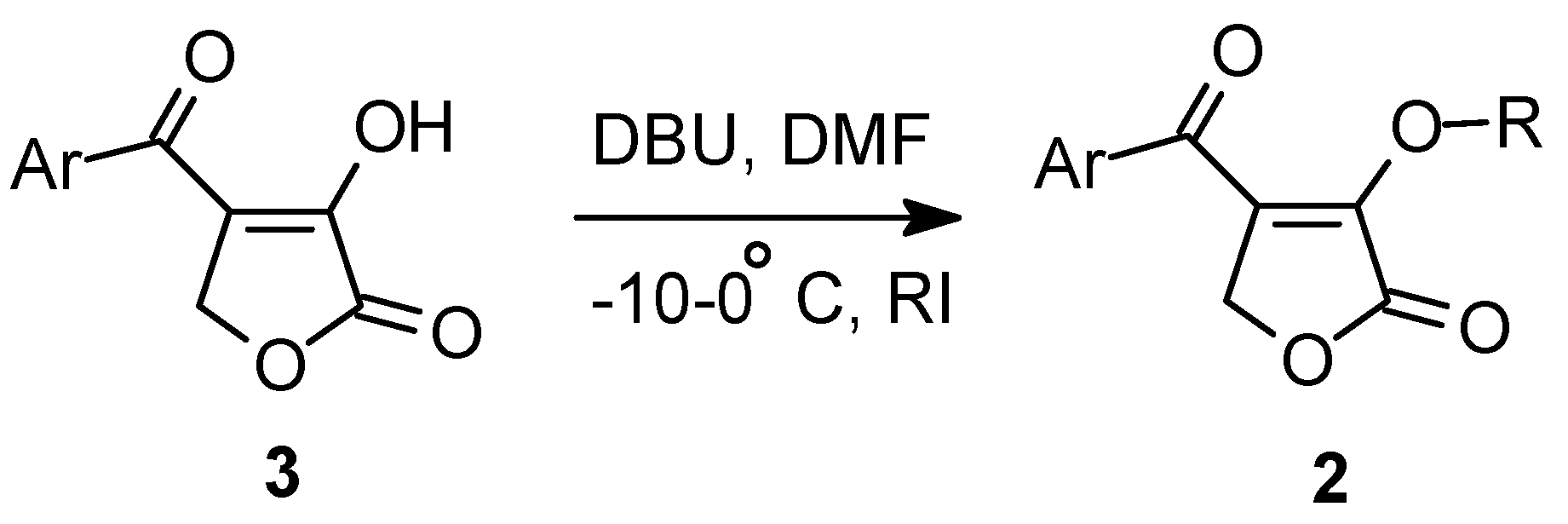
General Procedure: 4-Aroyl-3-alkoxy-2(5H)-furanones (2).
General Procedure: 7-Aryl-4,5-dihydro-2-oxo-3H,8H-furo[3,4-b][1,4]diazepines (1).
| Product | 1H NMR a δ (ppm) | 13C NMR a δ (ppm) | Molecularformula | MS | Analysis % Calc./Found | |
|---|---|---|---|---|---|---|
| 2a | 1.17 (t, 3H), 4.32 (q, 2H), 5.01 (s, 2H), 7.50, 7.63, 7.86 (m, m, d, 5H) | 14.85, 67.63, 67.85, 127.97, 128.18, 128.98, 133.58, 136.32, 142.79, 167.59, 189.49 | C13H12O4 | 232, 203, 188, 159, 143, 132, 105 | 67.24 | 5.01 |
| 2b | 4.78(d, 2H), 5.04 (s, 2H), 5.07 (d, 1H), 5.10 (d, 1H), 5.76 (m, 1H), 7.50, 7.63, 7.85 (m, m, d, 5H) | 67.72, 71.74, 118.97, 128.35, 129.15, 130.25, 131.60, 133.74, 136.36, 143.54, 167.67, 189.40 | C14H12O4 | 244, 172, 122, 105 | 68.85 | 4.95 |
| 2c | 1.01 (t, 3H), 4.47 (q, 2H), 5.05 (s, 2H), 7.40 (m, 4H) | 14.30, 66.28, 67.20, 126.32, 127,22, 127.89, 128.94, 130.03, 131.05, 138.10, 146.62, 166.95, 188.09 | C13H11ClO4 | 266, 231, 203, 166, 159, 139, 131 | 58.55 | 4.16 |
| 2d | 0.62 (d, 6H), 1.58 (m, 1H), 4.22 (d, 2H), 5.06 (s, 2H), 7.39 (m, 4H) | 18.19, 28.44, 66.79, 77.70, 126.87, 127.17, 128.34, 129.65, 130.88, 131.50, 138.89, 147.21, 167.54, 188.85 | C15H15ClO4 | 294, 239, 203, 159, 139, 131 | 61.13 | 5.13 |
| 2e | 4.13 (s, 3H), 5.08 (s, 2H), 7.62, 7.88, 8.07 (3m, 4H) | 59.03, 67.14, 112.65, 117.47, 126.59, 129.15, 132.53, 132.77, 136.07, 137.30, 145.31, 187.05 | C13H9NO4 | 243, 214, 168, 130, 102 | 64.20 | 3.73 |
| 2f | 2.38 (s, 3H), 3.87 (s, 3H), 4.94 (s, 2H), 7.23 (d, 2H), 7.71 (d, 2H) | b | C13H12O4 | 232, 189, 159, 119, 91 | b | |
| 2g | 1.11 (t, 3H), 2.35 (s, 3H), 4.21 (q, 2H), 4.91 (s, 2H), 7.21 (d, 2H), 7.70 (d, 2H) | 15.02, 21.60, 67.84, 68.01, 128.51, 129.11, 129.36, 133.88, 143.37, 145.05, 167.79, 189.19 | C14H14O4 | 246, 231, 189, 159, 146, 119, 91 | 68.27 | 5.73 |
| 2h | 0.80 (t, 3H), 1.19 (m, 2H), 1.51 (m, 2H), 2.45 (s, 3H), 4.27 (t, 2H), 5.03 (s, 2H), 7.29 (d, 2H), 7.77 (d, 2H) | 13.46, 18.56, 21.77, 31.52, 67.97, 72.06, 128.60, 129.19, 129.49, 134.17, 143.91, 145.05, 167.96, 189.39 | C16H18O4 | 275 (M+1), 219, 119, 91 | 70.06 | 6.62 |
| 2i | 0.85 (t, 3H), 1.21 (m, 8H), 1.52 (m, 2H), 2.44 (s, 3H), 4.27 (t, 2H), 5.03 (s, 2H), 7.29 (d, 2H), 7.67 (d, 2H) | 13.87, 21.64, 22.36, 25.22, 28.60, 29.41, 31.47, 67.85, 72.19, 128.60, 129.06, 129.40, 134.08, 143.83, 144.85, 167.88, 189.26 | C19H24O4 | 316, 301, 219, 146, 119, 91 | 72.11 | 7.65 |
| 2j | 2.43 (s, 3H), 5.02 (s, 2H), 5.39 (s, 2H), 7.10 (m, 2H), 7.25 (m, 5H), 7.69 (d, 2H) | 21.81, 68.01, 72.95, 127.88, 127.97, 128.43, 128.51, 129.23, 129.58, 130.58, 135.18, 143.29, 145.02, 168.05, 189.10 | C19H16O4 | 308, 278, 225, 187, 144, 119, 91 | 74.00 | 5.23 |
| 2k | 2.37 (s, 3H), 4.76 (d, 2H), 4.97 (s, 2H), 5.07 (bs, 1H), 5.12 (bs, 1H), 5.64 – 5.80 (m, 1H), 7.19 (d, 2H), 7.71 (d, 2H) | 21.60, 67.80, 71.77, 118.98, 129.10, 129.40, 129.61, 131.68, 133.78, 142.94, 144.97, 167.72, 188.89 | C15H14O4 | 258, 243, 146, 119, 91 | 69.74 | 5.47 |
| 2l | 0.76 (d, 6H), 1.81 (m, 1H), 2.44 (s, 3H), 4.07 (d, 2H), 5.04 (s, 2H), 7.28 (d, 2H), 7.77 (d, 2H) | 18.52, 21.81, 28.65, 67.97, 78.22, 128.77, 129.24, 129.52, 134.21, 144.17, 145.01, 168.05, 189.45 | C16H18O4 | 274, 259, 219, 174, 146, 119, 91 | 67.83c | 6.76c |
| 2m | 1.28 (t, 3H), 2.43 (s, 3H), 4.23 (q, 2H), 5.01 (s, 2H), 5.08 (s, 2H), 7.27 (d, 2H), 7.88 (d, 2H) | 14.01, 21.73, 61.60, 65.90, 68.01, 129.15, 129.70, 133.79, 141.89, 144.93, 167.71, 168.26, 188.67 | C16H16O6 | 305 (M+1), 277, 185, 123, 119 | 63.14 | 5.30 |
| 2n | 1.16 (d, 6H), 2.45 (s, 3H), 5.05 (s, 2H), 5.25 (qu, 1H), 7.27 (d, 2H), 7.89 (d, 2H) | 21.73, 22.45, 67.97, 74.93, 128.94, 129.61, 130.29, 133.88, 143.08, 144.85, 168.22, 189.28 | C15H16O4 | 261 (M+1), 219, 147, 119, 91 | 69.20 | 6.20 |
| 2o | 1.39 - 1.45 (m, 4H), 1.60 – 1.66 (m, 4H), 2.45 (s, 3H), 5.06 (s, 2H), 5.48 (t, 1H), 7.28 (d, 2H), 7.76 (d, 2H) | 21.69, 23.12, 33.12, 67.93, 84.26, 128.85, 129.44, 130.04, 134.04, 143.07, 144.63, 168.14, 189.27 | C17H18O4 | 286, 219, 119, 91 | 71.31 | 6.34 |
| 2p | 4.13 (s, 3H), 5.09 (s, 2H), 7.27 (m, 2H), 8.34 (m, 2H) | 59.13, 67.31, 123.41, 126.59, 129.95, 141.44, 145.78, 150.20, 167.14, 187.73 | C12H9NO6 | 263, 146, 150, 104 | 54.76 | 3.45 |
| 2q | 2.59 (s, 3H), 4.07 (s, 3H), 5.01 (s, 2H), 6.89 (d, 1H), 7.71 (d, 1H) | b | C11H10O4S | 238, 223, 140, 125, 112, 97 | b | |
| 4 | 2.41 (s, 6H), 4.98 (s, 4H), 5.08 (s, 4H), 7.04 (m, 2H), 7.19 (d, 4H), 7.62 (m, 2H), 7.77 (d, 4H) | 21.65, 67.88, 70.28, 128.79, 129.11, 129.28, 130.61, 133.79, 142.95, 145.02, 167.67 | C32H26O8 | 539 (M+1), 321, 195, 119 | 71.37 | 4.87 |
| Producta | Yield (%) | Mp (°C) | 1H NMRb δ (ppm) | Molecular formula | MS | Analysis % Calc./Found | ||
|---|---|---|---|---|---|---|---|---|
| 1c | 42 | 176 | 3.55 (bs, 2H), 3.94 (bs, 2H), 4.56 (bs, 2H), 6.39 (bs, 1H), 7.37 (m, 4H) | C13H11ClN2O2 | 262, 227, 217, 183 | 59.44 | 4.22 | 10.66 |
| 1e | 48 | 172 | 3.62 (bs, 2H), 4.21 (bs, 2H), 4.76 (s, 2H), 5.68 (bs, 1H), 7.55, 7.74 (2m, 4H) | C14H11N3O2 | 253, 252, 208, 181, 179, 142, 140, 102 | 66.40 | 4.38 | 16.59 |
| 1f | 39 | 178 | 2.38 (s, 3H), 3.61 (bs, 2H), 4.20 (bs, 2H), 4.78 (s, 2H), 5.31 (bs, 1H), 7.22 (m, 2H), 7.34 (m, 2H) | C14H14N2O2 | 242, 227, 197, 183, 170, 128, 105, 91 | 69.41 | 5.82 | |
| 1p | 51 | 198 | 3.57 (bs, 2H), 4.15 (bs, 2H), 4.64 (s, 2H), 5.51 (bs, 1H), 7.53 (d, 2H), 8.17 (d, 2H) | C13H11N3O4 | 273, 256, 228, 182, 151 | 57.14 | 4.06 | 15.38 |
| 1q | 27 | 170 | 2.47 (s, 3H), 3.69 (bs, 2H), 4.13 (bs, 2H), 5.10 (s, 2H), 5.72 (bs, 1H), 6.71 (d, 1H), 7.29 (d, 1H) | C12H12N2O2S | 248, 233, 215, 203, 176, 111 | 58.05 | 4.87 | 11.28 |
Acknowledgments
References and Notes
- Zimmer, H.; Nauss, J. L.; Amer, A. J. Heterocyclic.Chem. 1998, 35, 25–28. [CrossRef]
- Pigeon, P.; Decroix, B. Tetrahedron Lett. 1998, 39, 8659–8662, and references cited therein.
- Fray, M. J.; Bull, D. J.; Cooper, K.; Parry, M. J.; Slefaniak, H. M. J. Med. Chem. 1995, 38, 3524–3535.
- Kawakami, Y.; Kitani, H.; Yuasa, S.; Abe, M.; Moriwaki, M.; Kagoshima, M.; Tersawa, M.; Tahara, T. Eur. J. Med. Chem. 1996, 31, 683–692. [CrossRef]
- Rajappan, V. P.; Hosmane, R. S. Nucleosides Nucleotides 1998, 17, 1141–1151.
- Bertelli, L.; Biagi, G.; Giorgi, I.; Livi, O.; Manera, C. Farmaco 1998, 53, 305–311.
- Goerlitzer, K.; Wilpert, C.; Ruebsamen-Waigmann, H.; Suhartono, H; Wang, L.; Immelmann, A. Arch. Pharm. (Weinheim Ger.) 1995, 328, 247–255.
- Zimmer, H.; Amer, A.; Ho, D.; Palmer-Sungail, R. J. Heterocyclic Chem. 1991, 28, 1501–1510. [CrossRef]
- Amer, A.; Ventura, M.; Zimmer, H. J. Heterocyclic Chem. 1983, 20, 359–364, and references cited therein.
- Zimmer, H.; Palmer-Sungail, R.; Ho, D.; Amer, A. J. Heterocyclic Chem. 1993, 30, 161–167.
- Crystallographic data for the structures reported in this paper have been deposited with Cambridge Crystallographic Data Centre as supplementary publications no. CCDC 146275-146279. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; e-mail: [email protected]).
- Sample Availability: Not Available
© 2003 ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zimmer, H.; Librera, C.P.; Hausner, S.; Bauer, J.; Amer, A. Novel 4-Aroyl-3-alkoxy-2(5H)-furanones as Precursors for the Preparation of Furo[3,4-b][1,4]-diazepine Ring System. Molecules 2003, 8, 735-743. https://doi.org/10.3390/81000735
Zimmer H, Librera CP, Hausner S, Bauer J, Amer A. Novel 4-Aroyl-3-alkoxy-2(5H)-furanones as Precursors for the Preparation of Furo[3,4-b][1,4]-diazepine Ring System. Molecules. 2003; 8(10):735-743. https://doi.org/10.3390/81000735
Chicago/Turabian StyleZimmer, Hans, Christian P. Librera, Sven Hausner, Jeanette Bauer, and Adel Amer. 2003. "Novel 4-Aroyl-3-alkoxy-2(5H)-furanones as Precursors for the Preparation of Furo[3,4-b][1,4]-diazepine Ring System" Molecules 8, no. 10: 735-743. https://doi.org/10.3390/81000735




