Synthesis of New Cyano-Substituted bis-Benzothiazolyl Arylfurans and Arylthiophenes
Abstract
:Introduction
Results and Discussion
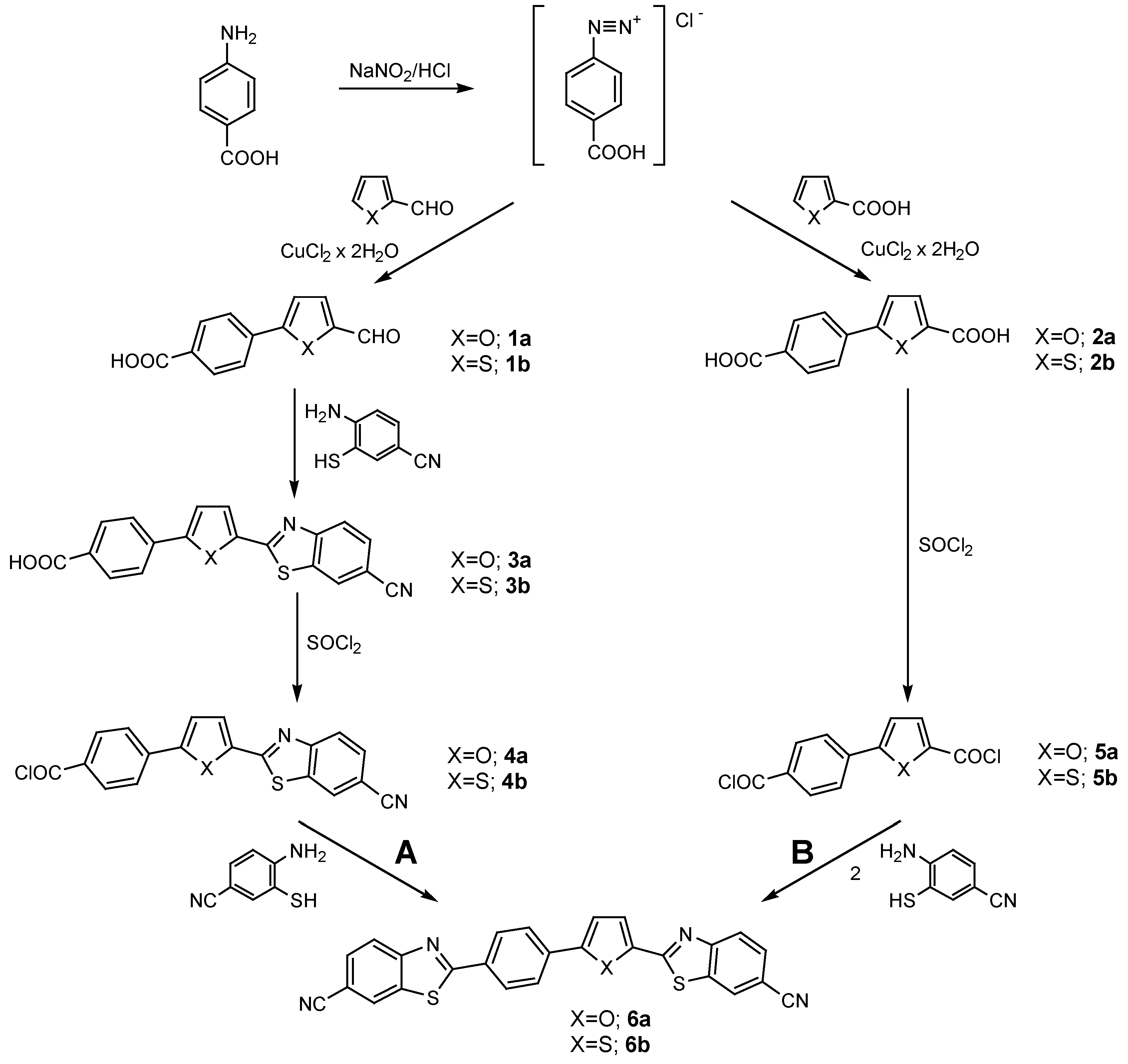
Experimental
General
General Procedure for the Arylation of Compounds 1a, 1b, 2a and 2b.
General Procedure for the Synthesis of Benzothiazolyl Compounds 3a and 3b.
General Procedure for the Synthesis of Chlorocarbonyl Compounds 4a, 4b, 5a and 5b.
General Procedure for the Synthesis of bis-Benzothiazolyl Compounds 6a and 6b.
Acknowledgments
References
- Lácová, M.; Chovancová, J.; Hýblová, O.; Varkonda, S. Synthesis and Pesticidal Activity of Acyl Derivatives of 4-chloro-2-aminobenzothiazole and the Products of their Reduction. Chem. Pap. 1991, 45, 411–418. [Google Scholar]
- Chulák, I.; Sutorius, V.; Sekerka, V. Benzothiazole compound XXXV. Synthesis of 3-substituted 2-benzylbenzothiazolium Salts and their Growth-regulating Effect on Triticum Aestivum L. Chem. Pap. 1990, 44, 131–138. [Google Scholar]
- Papenfuhs, T. Preparation of Benzothiazoles as Intermediates for Dyes, Plant Protectants and Pharmaceuticals. Ger. Offen. DE 3,528,032, 1987. [Google Scholar]
- Bradshaw, T.D.; Bibby, M.C.; Double, J.A.; Fichtner, I.; Cooper, P.A.; Alley, M.C.; Donohue, S.; Stinson, S.F.; Tomaszewjski, J. E.; Sausville, E.A.; Stevens, M.F.G. Preclinical Evaluation of Amino Acid Prodrugs of Novel Antitumor 2-(4-amino-3-methylphenyl)benzothiazoles. Mol. Cancer Therapeutics. 2002, 1, 239–246. [Google Scholar]
- Bradshaw, T.D.; Chua, M.S.; Browne, H.L.; Trapani, V.; Sausville, E.A.; Stevens, M.F.G. In Vitro Evaluation of Amino Acid Prodrugs of Novel Antitumor 2-(4-amino-3- methylphenyl)benzothiazoles. Br. J. Cancer. 2002, 86, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, I.; Jennings, S.A.; Vishnuvajjala, B.R.; Westwell, A.D.; Stevens, M.F.G. Antitumor Benzothiazoles. 16. Synthesis and Pharmacutical Properties of Antitumor 2-(4- aminophenyl)benzothiazole Amino Acid Prodrugs. J. Med. Chem. 2002, 45, 744–747. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeny, M.A. Synthesis of Certain Pyrimido[2,1-b]benzothiazole and Benzothiazolo[2,3- b]quinazoline Derivatives for in Vitro Antitumor and Antiviral Activities. Arzeneim. Forsch. 2000, 50, 848–853. [Google Scholar] [CrossRef]
- Racanè, L.; Tralić-Kulenović, V.; Fišer-Jakić, L.; Boykin, D.W.; Karminski-Zamola, G. Synthesis of bis-Substituted Amidinobenzothiazoles as Potential Anti-HIV Agents. Heterocycles. 2001, 55, 2085–2098. [Google Scholar]
- Sener, E.A.; Arpaci, O.T.; Yalcin, I.; Altanlar, N. Synthesis and Microbiological Activity of Some Novel 5-benzamido- and 5-phenylacetamido- Substituted 2-phenylbenzoxazole Derivatives. Farmaco. 2000, 55, 397–405. [Google Scholar] [CrossRef]
- Mruthyunjayaswamy, B.H.M.; Shanthaveerappa, B.K. Synthesis and Pharmacological Evaluation of 3,5-disubstituted indole-2-[N beta-(substituted benzopyran-2’-one-3’-carboxyl)]carboxy hydrazides and 2H-3-(various substituted indol-3’-yl)methyl-1,3-benzothiazoles. Indian J. Chem. Sect. B. 2000, 39, 433–439. [Google Scholar]
- Temiz-Arpaci, O.; Aki-Sener, E.; Yalcin, I; Altanlar, N. Synthesis and Antimicrobial Activity of Some 2-[p-substituted-phenyl]benzoxazol-5-yl-arylcarboxyamides. Arch. Pharm. Weinheim, Ger. 2002, 335, 283–288. [Google Scholar] [CrossRef]
- Delmas, F.; Di Giorgio, C.; Robin, M.; Azas, N.; Gasquet, M.; Detang, C.; Costa, M.; Timon- David, P.; Galy, J.P. In Vitro Activities of Position 2 Substitution–bearing 6-nitro and 6-amino- benzothiazoles and their Corresponding Anthranilic Acid Derivatives Against Leishmania Infantum and Trichomonas Vaginalis. Antimicrob. Ag. Chemother. 2002, 46, 2588–2594. [Google Scholar] [CrossRef]
- Pattan, S.R.; Babu, S.N.N.; Angadi, J. Synthesis and Biological Activity of 2-amino [5’-(4’- sulphonylbenzylidene)-2,4-thiazolidine dione]-7-(substituted)-6-fluoro benzothiazoles. Indian J. Heterocyclic Chem. 2002, 11, 333–334. [Google Scholar]
- Mahmood-ul-Hasan; Chohan, Z.H.; Supuran, C.T. Antibacterial Zn(II) Compounds of Schiff Bases Derived from Some Benzothiazoles. Main Group Met. Chem. 2002, 25, 291–296. [Google Scholar] [CrossRef]
- Akyama, S.; Ochiai, T.; Nakatsuji, S.; Nakashima, K.; Ohkura, Y. Preparation and Evaluation of Fatty Acid Esters of Fluorescent p-Substituted Phenols as Substrates for Measurement of Lipase Activity. Chem. Pharm. Bull. 1987, 35, 3029–3032. [Google Scholar] [CrossRef]
- Farcasan, V.; Balazs, I. Derivati ai Furanului (VII). Stud. Univ. Babes-Bolyai-Ser. Chem. 1968, 13, 123–127. [Google Scholar]
- Dotrong, M.; Mehta, R.; Balchin, G.A.; Tomlinson, R.C.; Sinsky, M.; Lee, C.Y.-C.; Evers, R.C. Synthesis, Processing, and Third-Order Nonlinear Optical Properties of Benzobisthiazole Polymers Containing Thiophene Moieties. J. Pol. Sci. Part A: Polym. Chem. 1993, 31, 723–729. [Google Scholar] [CrossRef]
- Racanè, L.; Tralić-Kulenović, V.; Karminski-Zamola, G.; Fišer-Jakić, L. Synthesis and Fluorescent Properties of Some New Unsymmetric bis-Benzothiazolyl Furans and Thiophenes. Monatsh. Chem. 1995, 126, 1375–1381. [Google Scholar] [CrossRef]
- Kornilov, M.Y.; Ruban, E.M.; Fredchuk, V.N.; Starinskaya, E.V.; Buznik, M.V. Reaction of Chromophores in Asymetric 2,5-disubstituted Derivatives of Furan and Thiophene. Zh. Org. Khim. 1973, 9, 2577–2582. [Google Scholar]
- Malinowski, S. Reaction of Diazo Compounds with Unsaturated Compounds II. Arylation of furfural. Polish J. Chem. 27, 54–61.
- Tralić-Kulenović, V.; Fišer-Jakić, L.; Lazarević, Z. Synthesis and Absorption Spectral Properties of Substituted Phenylfurylbenzothiazoles and their Vinylogues. Monatsh. Chem. 1994, 125, 209–215. [Google Scholar] [CrossRef]
- Tralić-Kulenović, V.; Karminski-Zamola, G.; Racanè, L.; Fišer-Jakić, L. Synthesis of Symmetric and Unsymmetric bis-Cyanosubstituted Heterocycles. Heterocyclic Comm. 1998, 4, 423–428. [Google Scholar]
- Bogert, M.T.; Naiman, B. Research on Thiazoles XX. J. Am. Chem. Soc. 1935, 57, 1529–1533. [Google Scholar] [CrossRef]
- Sample availability: Samples of compounds 2b, 3b, 6a and 6b are available from MDPI.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Racanè, L.; Tralić-Kulenović, V.; Boykin, D.W.; Karminski-Zamola, G. Synthesis of New Cyano-Substituted bis-Benzothiazolyl Arylfurans and Arylthiophenes. Molecules 2003, 8, 342-348. https://doi.org/10.3390/80300342
Racanè L, Tralić-Kulenović V, Boykin DW, Karminski-Zamola G. Synthesis of New Cyano-Substituted bis-Benzothiazolyl Arylfurans and Arylthiophenes. Molecules. 2003; 8(3):342-348. https://doi.org/10.3390/80300342
Chicago/Turabian StyleRacanè, Livio, Vesna Tralić-Kulenović, David W. Boykin, and Grace Karminski-Zamola. 2003. "Synthesis of New Cyano-Substituted bis-Benzothiazolyl Arylfurans and Arylthiophenes" Molecules 8, no. 3: 342-348. https://doi.org/10.3390/80300342



