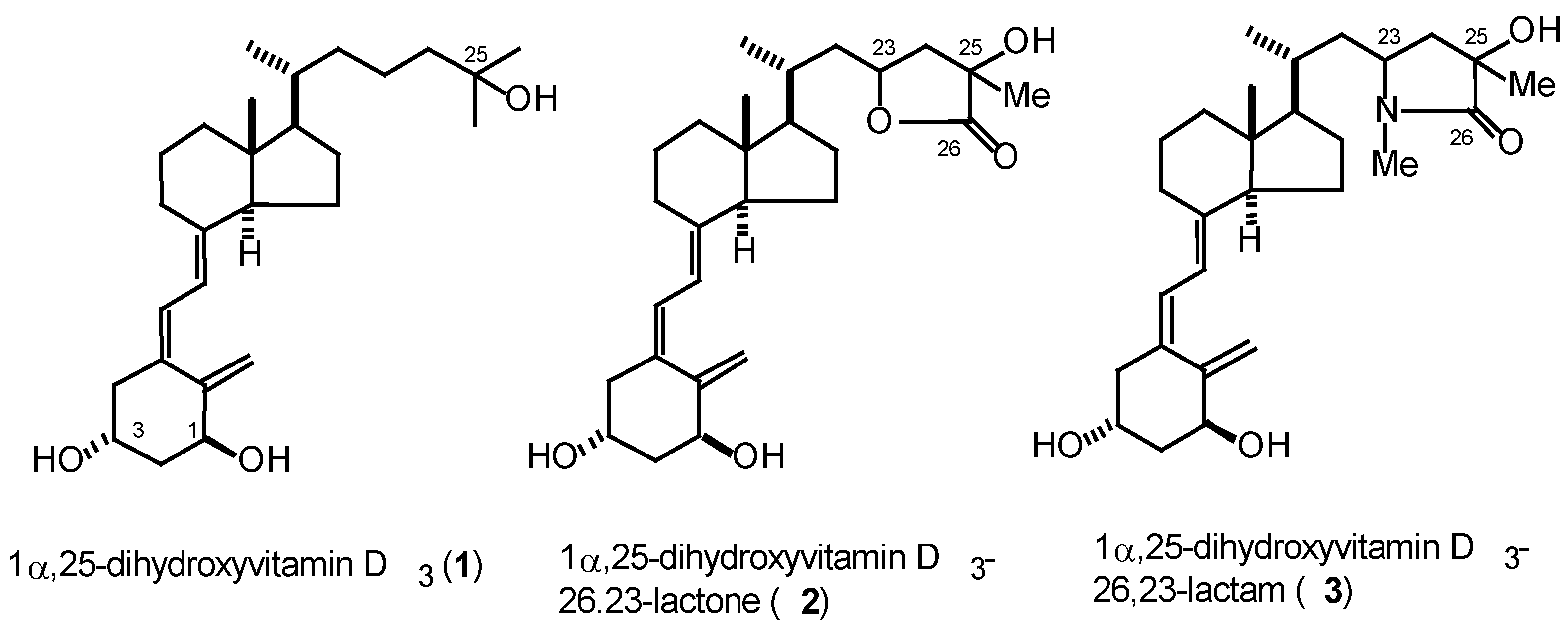
General
Optical rotations were recorded with a JASCO DIP-370 polarimeter. IR spectra were measured with a JASCO VALOR-III FT-IR spectrophotometer. 1H- and 13C-NMR spectra were recorded on JNM-α500 instruments at 500 and 125 MHz, respectively. Mass spectra were recorded on JEOL JMA-HX110 spectrometers.
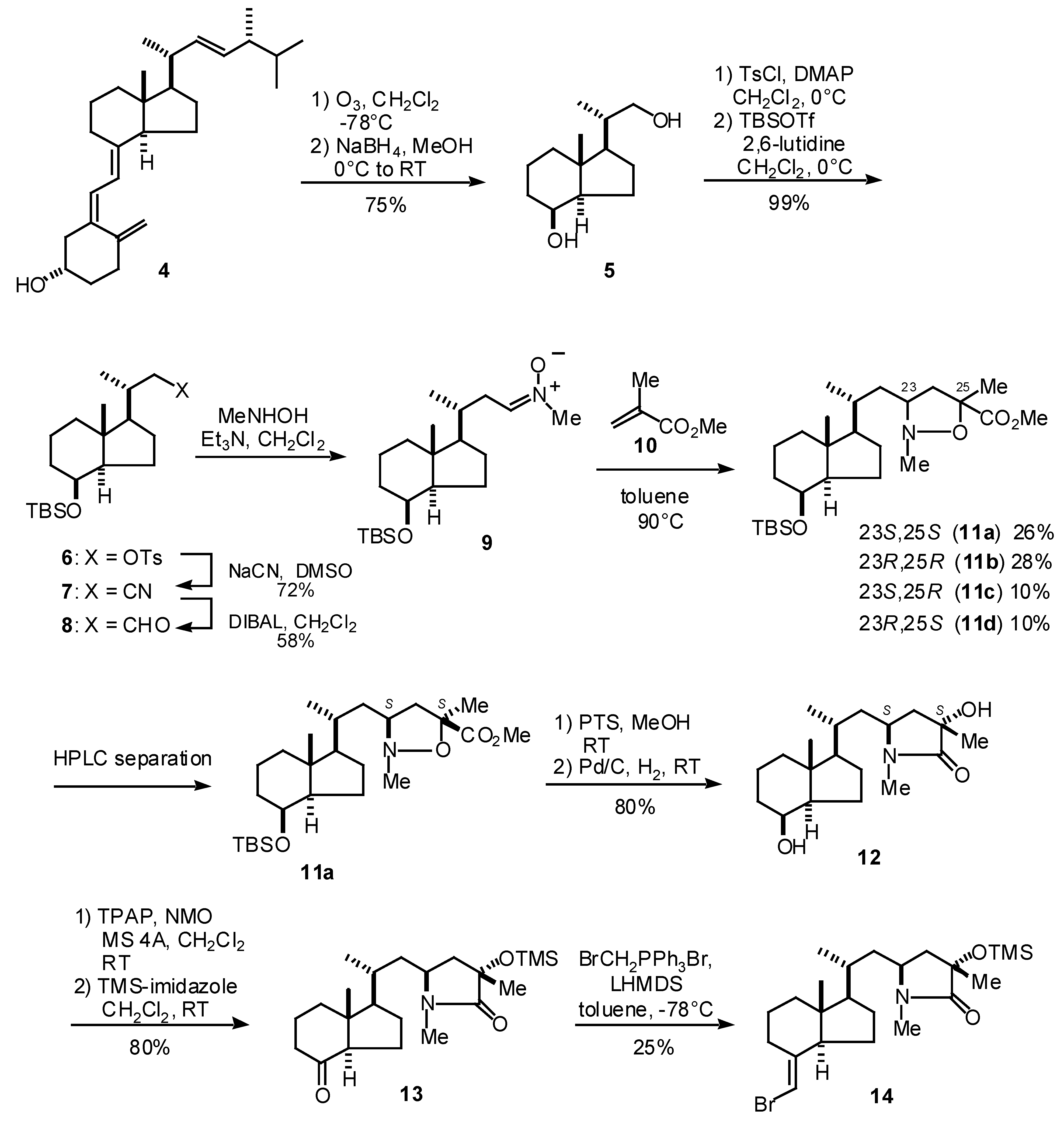
(8S,20R)-De-A,B-8-Hydroxy-20-(hydroxymethyl)pregnane (5). Vitamin D2 (4) (5.00 g, 12.63 mmol) was dissolved in CH2Cl2 (170 mL), and MeOH (70 mL) was added. After cooling to –78°C, a stream of ozone was passed to the solution for 1 h. The remaining ozone was purged with a stream of N2. After warming up to 0°C, NaBH4 (1.43g, 37.8 mmol) was added and stirred for 1h. The reaction mixture was then acidified by adding 2N HCl and extracted with CH2Cl2. The organic layer was washed with water, dried over MgSO4, filtered and concentrated in vacuo. The residue was chromatographed on silica gel (6:1 hexane - ethyl acetate) to give diol 5 (2.8g, 75%) as a white solid. 1H-NMR (CDCl3) δ 4.09 (d, J = 2.6 Hz, 1H), 3.64 (dd, J = 3.3, 10.6 Hz, 1H), 3.39 (dd, J = 6.6, 10.6 Hz, 1H), 1.99 (dd, J = 2.6, 13.2 Hz, 1H), 1.12~1.92 (m, 14H), 1.03 (d, J = 6.6 Hz, 3H), 0.002 (s, 3H).
(8S,20R)-De-A,B-8-(tert-Butyldimethylsilyl)oxy-20-[(p-tolylsulfonyl)oxymethyl]pregnane (6). DMAP (3.7 g, 30.2 mmol) and p-TsCl (3.8 g, 19.63 mmol) was added to a solution of 5 (3.2 g, 15.10 mmol) in CH2Cl2 (45 mL). After stirring overnight at room temperature, the reaction mixture was poured into water (110 mL) and extracted with CH2Cl2. The organic layer was washed with water, dried over MgSO4, filtered and concentrated in vacuo. The residue was dissolved in CH2Cl2 (70 mL) and then 2,6-lutidine (4.5 mL, 38.99 mmol) and TBSOTf (5.3 mL, 23.11 mmol) was added. After stirring at room temperature for 30 min, the solution was cooled to 0°C, satuated NaHCO3 was added and extracted with CH2Cl2. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was chromatographed on silica gel (hexane) to give 5 as a colorless oil (4.29 g, 99%). 1H-NMR (CDCl3) δ 9.72 (dd, J = 1.3, 3.3 Hz, 1H), 3.99 (d, J = 2.6 Hz, 1H), 2.43 (m, 1H), 1.93 (m, 1H), 1.78 (m, 2H), 1.66 (m, 1H), 1.56 (m, 1H), 1.35 (m, 3H), 1.25 (m, 2H), 1.11 (m, 2H), 1.00 (d, J = 6.3 Hz, 3H), 0.96 (s, 3H), 0.89 (s, 9H), 0.01 (s, 3H), 0.002 (s, 3H).
(8S,20R)-De-A,B-8-(tert-Butyldimethylsilyl)oxy-20-(cyanomethyl)pregnane (7). To a solution of 6 (2.00 g, 4.17 mmol) in DMSO (30 mL) was added NaCN (270 mg, 5.42 mmol) and the resulting mixture was heated at 90°C for 1 h. The reaction mixture was poured into water (60 mL) and the organics were extracted with ethyl acetate. The extracts were washed with water and brine, dried over MgSO4, filtered and concentrated in vacuo. The residue was chromatographed on silica gel (hexane) to give 7 (1.02 g, 72%) as a colorless oil. 1H-NMR (CDCl3) δ 4.01 (d, J = 2.6 Hz, 1H), 2.34 (dd, J = 4.0, 16.5 Hz, 1H), 2.23 (dd, J = 6.6, 16.5 Hz, 1H), 1.10~2.00 (m, 20H), 1.14 (d, J = 6.6 Hz, 3H), 0.93 (s, 3H), 0.89 (s, 9H), 0.02 (s, 3H), 0.00 (s, 3H).
(8S,20R)-De-A,B-8-(tert-Butyldimethylsilyl)oxy-20-(formylmethyl)pregnane (8). To a solution of DIBAH (1M in hexane) (6.1 mL, 6.1 mmol) in CH2Cl2 (20 mL) was added a solution of 7 (1.02 g, 3.05 mmol) in CH2Cl2 (25 mL) at 0°C with dropwise and the mixture was stirred for 1 h. The reaction mixture was quenched by the addition of saturated NH4Cl solution, and the resulting mixture was diluted with ether, and then stirred for another 30 min. The mixture was dried over MgSO4 and filtered through a pad of Celite, and the filtrates were concentrated in vacuo. The residue was chromatographed on silica gel (hexane) to give aldehyde 8 (595 mg, 58%) as a colorless oil. 1H-NMR (CDCl3) δ 9.75 (dd, J = 1.3, 3.3 Hz, 1H), 4.00 (d, J = 2.6 Hz, 1H), 2.46 (dd, J = 2.0, 13.5 Hz, 1H), 1.03~2.20 (m, 20H), 1.00 (d, J = 6.3 Hz, 3H), 0.96 (s, 3H), 0.89 (s, 9H), 0.01 (s, 3H), 0.002 (s, 3H).
(8S,20R)-De-A,B-8-(tert-Butyldimethylsilyl)oxy-20-[4-(2,5-dimethyl-5-isoxazolidinecarboxylic acid methyl ester)methyl]pregnane (11). To a mixture of 8 (595 mg, 2.66 mmol) in CH2Cl2 (8 mL) was added N-methyl hydroxylamine hydrochloride (184 mg, 3.19 mmol) and Et3N (1.0 mL), and the resulting mixture was stirred at room temperature for 3 h. To the reaction mixture was added saturated NaHCO3, and the mixture was extracted with CH2Cl2. The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was dissolved in toluene (17 mL) and methyl methacrylate (10) (1.0 mL, 9.32 mmol) was added, and the resulting mixture was heated at 90°C for 2 h. The reaction mixture was concentrated in vacuo, and the residue was purified by HPLC using a PEGASIL Silica 60-5 column (ϕ 20x150 mm, Senshu Pak.) and elution with 15% ethyl acetate in hexane to give 11a (316.6 mg, 26%), 11b (343.6 mg, 28%), 11c (116.5 mg, 10%) and 11d (125.5 mg, 10%), respectively.
11a (23S, 25S): [α]24D = +112.4° (c 1.1, CHCl3); 1H-NMR (CDCl3) δ 3.98 (br s, 1H), 3.76 (s, 3H), 2.67 (s, 3H), 2.57 (br s, 1H), 2.46 (dd, J = 3.0, 13.1 Hz, 1H), 2.24 (dd, J = 7.0, 13.1 Hz, 1H), 1.94 (br d, J = 13.1 Hz, 1H), 1.79 (m, 2H), 1.65 (m, 2H), 1.54 (m, 1H), 1.47 (s, 3H), 1.39 ( m, 4H), 1.21 (m, 2H), 1.11 (m, 2H), 1.01 (m, 1H), 0.92 (d, J = 8.2 Hz, 3H), 0.91 (s, 3H), 0.88 (s, 6H), -0.01 (d, J = 7.0 Hz, 9H); 13C-NMR (CDCl3) δ 175.67, 77.25, 76.99, 76.74, 69.36, 66.95, 57.37, 53.03, 52.57, 45.87, 43.27, 42.17, 40.73, 38.06, 34.35, 34.03, 27.61, 25.77, 24.37, 23.01, 18.85, 17.99, 17.61, 13.74, -4.82, -5.21; IR (neat) 3318, 2952, 2856, 1738 cm-1; m/z 468 (M+H)+.
11b (23R, 25R): [α]24D = -26.7° (c 1.0, CHCl3); 1H-NMR (CDCl3) δ 3.98 (br s, 1H), 3.75 (s, 3H), 2.68 (s, 3H), 2.57 (dd, J = 9.0, 12.2 Hz, 1H), 2.51 (br s, 1H), 2.30 (m, 1H), 1.94 (br d, J = 12.2 Hz, 1H), 1.80 (m, 2H), 1.64 (m, 2H), 1.52 (m, 1H), 1.47 (s, 3H), 1.33 ( m, 3H), 1.23 (m, 3H), 1.13 (m, 2H), 1.00 (m, 1H), 0.92 (d, J = 6.0 Hz, 3H), 0.90 (s, 3H), 0.87 (s, 6H), -0.01 (d, J = 7.1 Hz, 9H); 13C-NMR (CDCl3) δ 175.69, 69.35, 68.00, 57.42, 52.97, 52.54, 47.64, 43.93, 42.22, 40.66, 38.83, 34.52, 34.34, 27.56, 25.77, 24.29, 23.02, 19.60, 17.99, 17.60, 13.65, -4,83, -5.21; IR (neat) 3287, 2951, 2857, 1738 cm-1; m/z 468 (M+H)+.
11c (23S, 25R): [α]24D = +62.5° (c 1.0, CHCl3); 1H-NMR (CDCl3) δ 3.98 (br s, 1H), 3.76 (s, 3H), 2.85 (dd, J = 6.1, 13.1 Hz, 1H), 2.70 (s, 3H), 2.00 (m, 1H), 1.86 (m, 1H), 1.66 (br d, J = 12.2 Hz, 1H), 1.57-1.42 (m, 1H), 1.52 (s, 3H), 1.37-0.99 (m, 12H), 0.91 (s, 3H), 0.89 ( d, J = 16.3 Hz, 3H), 0.88 (s, 6H), -0.01 (d, J = 7.2 Hz, 9H); 13C-NMR (CDCl3) δ 174.47, 77.24, 76.99, 76.74, 69.36, 66.78, 57.31, 53.02, 52.50, 46.82, 43,84, 42.18, 40.69, 38.38, 37.60, 34.34, 34.14, 29.66, 27.66, 25.77, 24.92, 24.83, 23.00, 22.62, 19.05, 18.90, 18.00, 17.60, 14.08, 13.72, -4.81, -5.21; IR (neat) 3790, 2951, 2856, 2777, 1739 cm-1; m/z 468 (M+H)+.
11d (23R, 25S): [α]24D = +16.9 (c 1.1, CHCl3); 1H-NMR (CDCl3) δ 3.98 (br s, 1H), 3.76 (s, 3H), 2.91 (dd, J = 6.1, 12.1 Hz, 1H), 1.95-1.91 (m, 2H), 1.81-1.76 (m, 2H), 1.70-1.64 (m, 2H), 1.59-1.47 (m, 2H), 1.51 (s, 3H), 1.37-1.20 (m, 6H), 1.12-0.97 (m, 3H), 0.92 (d, J = 9.2 Hz, 3H), 0.91 (s, 3H), 0.88 (s, 6H), -0.01 (d, J = 7.1 Hz, 9H); 13C-NMR (CDCl3) δ 174.84, 77.25, 76.99, 76.74, 69.38, 67.03, 57.42, 53.01, 52.48, 47.77, 44.88, 42.24, 40.68, 39.26, 34.37, 34.06, 31.56, 29.67, 27.51, 25.78, 24.86, 23.03, 22.62, 19.39, 18.00, 17.61, 14.08, 13.68, -4.81, -5.19; IR (neat) 3307, 2951, 2856, 2360, 1738 cm-1; m/z 468 (M+H)+.
(8S,20R,23S,25S)-De-A,B-8,25-Dihydroxy-cholestane-26,23-N-methyl lactam (12). To a solution of 11a (316.6 mg, 0.68 mmol) in MeOH (25 mL) was added a catalytic amount of p-TsOH and the resulting mixture was stirred for 12 h. The reaction mixture was diluted with ethyl acetate and the saturated NaHCO3 was added. The resulting mixture was extracted with ethyl acetate and the organics was washed with brine, dried over MgSO4, filtered and concentrated in vacuo. The residue was chromatographed on silica gel (5:1 hexane:ethyl acetate) to give the alcohol (218.7 mg, 91%) as a colorless oil. [α]24D = +145.9 (c 1.0, CHCl3); 1H-NMR (CDCl3) δ 3.98 (br s, 1H), 3.68 (s, 3H), 2.58 (s, 3H), 2.49 (br s, 1H), 2.38 (dd, J = 9.0, 13.0 Hz, 1H), 2.15 (dd, J = 8.2, 13.0 Hz, 1H), 1.90 (br d, J = 13.0 Hz, 1H), 1.80-1.69 (m, 3H), 1.54-0.93 (m, 11H), 1.39 (s, 3H), 0.84 (d, J = 6.3 Hz, 3H), 0.84 (s, 3H); 13C-NMR (CDCl3) δ 175.71, 69.26, 66.90, 57.24, 52.60, 52.59, 45.83, 43.28, 41.91, 40.41, 38.06, 34.03, 33.54, 27.45, 24.36, 22.47, 18.77, 17.38, 13.51; IR (neat) 3526, 2948, 2867, 2360, 1737 cm-1; m/z 354 (M+H)+. A mixture of the alcohol (218.7 mg, 0.62 mmol) and 10% Pd/C (100 mg) in EtOH (15 mL) was stirred under H2 atmosphere for 12 h. The reaction mixture was filtered through a pad of Celite and the filtrates were concentrated in vacuo to give 12 (180 mg, 90%) as a colorless oil. [α]24D = +145.9° (c 1.0, CHCl3); 1H-NMR (CDCl3) δ 3.98 (br s, 1H), 3.68 (s, 3H), 2.58 (s, 3H), 2.49 (br s, 1H), 2.38 (dd, J = 9.0, 13.1 Hz, 1H), 2.15 (dd, J = 8.0, 13.1 Hz, 1H), 1.90 (br d, J = 13.1 Hz, 1H), 1.80-1.69 (m, 3H), 1.54-0.93 (m, 11H), 1.39 (s, 3H), 0.84 (d, J = 6.0 Hz, 3H), 0.84 (s, 3H); 13C-NMR (CDCl3) δ 175.71, 69.26, 66.90, 57.24, 52.60, 52.59, 45.83, 43.28, 41.91, 40.41, 38.06, 34.03, 33.54, 27.45, 24.36, 22.47, 18.77, 17.38, 13.51; IR (neat) 3381, 2936, 2867, 2242, 1680 cm-1; m/z 324 (M+H)+.
(20R,23S,25S)-De-A,B-25-Trimethylsilylhydroxy-cholestane-8-one-26,23-N-methyl lactam (13). To a solution of 12 (212 mg, 0.66 mmol) in CH2Cl2 (5 mL) was added tetra-n-propylammonium perruthenate (12 mg, 0.66 mmol), 4-methylmorpholine N-oxide (310 mg, 2.64 mmol) and molecular sieves 4A powder, and the whole mixture was stirred for 2 h at room temperature. The reaction mixture was loaded on the silica gel column chromatography (1:2 hexane - ethyl acetate) to give the ketone (219 mg, quant. yield) as a colorless oil. A mixture of the ketone (95 mg, 0.3 mmol) and TMS-imidazole (0.22 mL, 1.5 mmol) in CH2Cl2 (5 mL) was stirred for 12 h at room temperature. The reaction mixture was cooled at 0°C and water was added to the mixture. The organic layer was extracted with CH2Cl2, and the extracts were dried over MgSO4, filtered and concentrated in vacuo. The residue was chromatographed on silica gel (2:1 hexane - ethyl acetate) to give 13 (107 mg, 91%) as a colorless oil. [α]24D = +36.0° (c 1.0, CHCl3); 1H-NMR (CDCl3) δ 3.55(m, 1H), 2.78 (s, 3H), 2.46 (dd, J = 8.0, 12.0 Hz, 1H), 2.32-1.20 (m, 19H), 1.02 (d, J = 6.1 Hz, 3H), 0.66 (s, 3H), 0.11 (s, 9H); 13C-NMR (CDCl3) δ 211.49, 174.56, 76.24, 61.95, 57.11, 53.90, 49.81, 43.41, 40.89, 39.74, 39.03, 32.47, 27.94, 27.70, 25.54, 23.96, 19.04, 18.68, 12.53, 1.75; IR (neat) 3035, 2957, 2360, 1705 cm-1; m/z 394 (M+H)+.
(7E)-(20R,23S,25S)-De-A,B-8-Bromomethylidene-25-trimethylsilylhydroxy-cholestane-26,23-N-methyl lactam (14). To an emulsion of bromomethyltriphenylphosphonium bromide (327 mg, 0.75 mmol) in toluene (2 mL) was added lithium hexamethyldisilazane (1 M in THF) (0.74 mL, 0.74 mmol) at -78°C and the mixture was warmed up to 0°C and stirred for 10 min. After cooling to -78°C, to the resulting solution was added 13 (60.1 mg, 0.15 mmol) in toluene (1 mL) with dropwise, and the mixture was stirred at the same temperature for 2 h. To the reaction mixture was added saturated NH4Cl and warmed up to room temperature. The resulting mixture was extracted with ethyl acetate and the organic layer was washed with brine, dried over MgSO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane) to give 14 (8.4 mg, 12%) and 13 (32 mg, 52%) was recovered. [α]24D = +79.47° (c 0.9, CHCl3); 1H-NMR (CDCl3) δ 5.56 (s, 1H), 3.45 (m, 1H), 2.80 (m, 1H), 2.68 (s, 3H), 2.19 (dd, J = 6.0, 12.2 Hz, 1H), 1.94-1.10 (m, 18H), 0.90 (d, J = 6.0 Hz, 3H), 0.49 (s, 3H), 0.02 (s, 9H); 13C-NMR (CDCl3) δ 174.79, 144.73, 97.70, 56.27, 55.89, 53.93, 45.52, 43.38, 39.90, 39.80, 32.92, 30.97, 29.69, 28.01, 27.70, 25.58, 22.48, 21.98, 18.76, 11.87; IR (neat) 3734, 2952, 1700, 1635, 1507 cm-1; m/z 470, 472 (M+H)+.
(2S)-2,4-Diol butanol anisilidene (17). To a solution of boranemethyl sulfide complex (100 mL, 1 mol) in THF (200 mL) was added the solution of (S)-malic acid (15) (50 g, 373 mmol) in THF (400 mL) at 0°C with dropwise over 3 h, and the resulting mixture was stirred for another 12 h at room temperature. The reaction mixture was re-cooled to 0°C and MeOH (400 mL) was added. The organics were evaporated in vacuo and the residue was dissolved in MeOH (300 mL) and evaporated in vacuo. The same sequences (addition of MeOH and concentration of organics) were repeated three times. The residue was dissolved in MeOH (300 mL) and p-TsOH (200 mg) was added, and the mixture was evaporated in vacuo to give 16 (50.63 g, 99%) as a colorless oil. To a solution of 16 (15 g, 141 mmol) in MeOH (240 mL) was added CSA (3.3 g, 14 mmol) and the mixture was heated at 90°C for 1 h. The resulting mixture was cooled to room temperature, and the organics were evaporated in vacuo. The residue was dissolved in CH2Cl2 and 4-methoxybenzaldehyde dimethylacetal (30 mL, 170 mmol) was added to the solution, and the resulting mixture was stirred for 6 h. To the reaction mixture was added saturated NaHCO3, and the organic layer was extracted with ethyl acetate and washed with brine. The aqueous layer was extracted twice with ethyl acetate. The combined organic layers were dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (2:1 hexane - ethyl acetate) to give 17 (18.23 g, 26%) as a pale yellow oil.
(3S)-3,5-Diol-1-pentene anisilidene (18). To a solution of (COCl)2 (6.0 mL, 67.4 mmol) in CH2Cl2 ( 50 mL) was added DMSO (12.0 mL, 168.5 mmol) dropwise at –78°C and the mixture was stirred for 15 min. To the resulting mixture was added 17 (8.23 g, 33.7 mmol) in CH2Cl2 (100 mL) and the mixture was stirred for 40 min. Et3N (470 mL, 337 mmol) was added and whole mixture was warmed to room temperature, and then stirred for another 30 min. Water (20 mL) was added and the organic layer was extracted with ethyl acetate. The extracts were washed with brine, dried over MgSO4, filtered and evaporated in vacuo. The residue in THF solution was added to a solution of methylphosphoniumylide in THF prepared from methylphosphonium bromide (16.6 g, 46.5 mmol) and n-BuLi (1.5 M in hexane, 30 mL, 45 mmol) in THF (250 mL) at 0°C and the whole mixture was stirred at 0°C for 30 min and at room temperature for another 1 h. After cooling to 0°C, water was added, and the organics were extracted with ethyl acetate. The extracts were washed with brine, dried over MgSO4, filtered and evaporated in vacuo. The residue was chromatographed on silica gel (10:1 hexane - ethyl acetate) to give 18 (2.77 g, 38%) as a colorless oil. 1H-NMR (CDCl3) 7.44 (d, J = 10.0 Hz, 2H), 6.88 (d, J = 10.0 Hz, 2H), 5.99 (m, 1H), 5.58 (s, 1H), 5.38 (dd, J = 13.1, 1.0 Hz, 1H), 5.19 (dd, J = 13.1, 1.0 Hz, 1H), 4.36 (dd, J = 12.2, 6.3 Hz, 1H), 4.28 (m, 1H), 4.00 (m, 1H), 3.80 (s, 3H), 1.92 (m, 1H), 1.60 (m, 1H).
(3S)-3-Methoxyphenylmethylyloxy-5-hydroxy-1-pentene (19). To a solution of 18 (2.77 g, 12.6 mmol) in CH2Cl2 (130 mL) was added DIBAH (0.95 M in hexane, 66 mL, 63 mmol) dropwise at -20°C and the mixture was stirred for 2 h. 2-Propanol was added, then water and silica gel. The resulting slurry was diluted with ethyl acetate and stirred at room temperature for another 1 h. The slurry was filtered through a pad of Celite and the filtrates were concentrated in vacuo. The residue was purified by silica gel column chromatography (10:1 hexane - ethyl acetate) to give 19 (1.15 g, 41%) as a colorless oil. 1H-NMR (CDCl3) δ 7.14 (d, J = 9.3 Hz, 2H), 6.77 (d, J = 9.3 Hz, 2H), 5.68 (m, 1H), 5.17 (m, 2H), 4.46 (d, J = 12.2 Hz, 2H), 4.20 (d, J = 12.2 Hz, 2H), 3,90 (m, 1H), 3.70 (s, 3H), 3.65 (m, 1H), 1.75 (m, 1H), 1.67 (m, 1H).
(3S)-3-[(Methoxyphenylmethylyl)oxyl-5-formyl-1-pentene (20). A mixture of 19 (1.15 g, 5.17 mmol) , NaHCO3 (1.7 g, 20.68 mmol) and Dess-Martin periodinane (4.4 g, 10.34 mmol) in CH2Cl2 (45 mL) was stirred for 1 h. The reaction mixture was directly loaded on a silica gel column (10:1 hexane - ethyl acetate) and purified to give 20 (990 mg, 89%) as a colorless oil. 1H-NMR (CDCl3) δ 9.48 (s, 1H), 7.15 (d, J = 10.1 Hz, 2H), 6.77 (d, J = 10.1 Hz, 2H), 5.50 (m, 1H), 5.04 (d, J = 17.4 Hz, 1H), 4.97 (d, J = 10.1 Hz, 1H), 4.41 (d, J = 13.2 Hz, 1H), 4.19 (d, J = 13.2 Hz, 1H), 4.00 (m, 1H), 2.35 (m, 1H), 2.2 (m, 1H).
(3S)-3-[(Methoxyphenylmethylyl)oxyl]-5-hydroxy-1-octen-7-yne (21). To a mixture of Mg (220 mg, 2.25 mmol) and HgCl2 (25 mg, 0.09 mmol) in ether (40 mL) was added propargyl bromide (0.75 mL, 9.9 mmol) at room temperature until the Mg was dissolved. The mixture was cooled to 0°C, and 20 (495 mg, 2.25 mmol) in ether (25 mL) was added dropwise. After being stirred for 15 min, saturated NH4Cl solution was added to the reaction mixture. The organics were extracted with ethyl acetate and washed with brine. The extracts were dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (8:1 hexane - ethyl acetate) to give 21 (393.1 mg, 67%) as a colorless oil.
(3S,5R)-3,5-Dihydroxy-1-octen-7-yne (23a). To a solution of 21 (741.5 mg, 2.8 mmol) in CH2Cl2 (50 mL) was added DDQ (953 mg, 4.2 mmol) at room temperature. After being stirred for 1 h, the reaction mixture was quenched by the addition of saturated NaHCO3. The mixture was extracted with CH2Cl2, dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (8:1 hexane - ethyl acetate) to give 23a (98.7 mg, 25%) and 22b (189 mg, 26%) as colorless oils. 23a: 1H-NMR (CDCl3) δ 5.94 (m, 1H), 5.31 (d, J = 10.2 Hz, 2H), 5.17 (d, J = 10.2 Hz, 2H), 4.49 (m, 1H), 4.41 (m, 1H), 4.11 (m, 1H), 4.05 (m, 1H), 2.41 (m, 2H), 2.16 (m, 1H), 1.86 (m, 1H), 1.73 (m, 1H).
(3S,5R)-3,5-Di[(t-butyldimethylsilyl)oxyl]-1-octen-7-yne (24). To a solution of 23a (98.7 mg, 0.70 mmol) in CH2Cl2 (7 mL) was added 2,6-lutidine (0.25 mL, 2.1 mmol) and TBSOTf (0.4 mL, 1.75 mmol) at 0°C and the mixture was stirred for 30 min at room temperature. The reaction mixture was re-cooled to 0°C, and saturated NaHCO3 was added. The organics were extracted with CH2Cl2 and the extracts were dried over MgSO4, filtered and concentrated in vacuo. The residue was chromatographed on silica gel (hexane) to give 24 (248.2 mg, 96%) as a colorless oil. [α]24D = -5.19° (c 0.7, CHCl3); 1H-NMR (CDCl3) δ 5.57 (m, 1H), 5.04 (d, J = 17.4 Hz, 1H), 4.94 (d, J =10.1 Hz, 1H), 4.11 (m, 1H), 3.82 (m, 1H), 2.25 (m, 2H), 1.87 (m, 1H), 1.81-1.53 (m, 2H), 1.44 (s, 1H), 0.78 (m, 18H), 0.03 (m, 12H); 13C-NMR (CDCl3) δ 141.90, 114.25, 81.47, 71.65, 70.07, 68.13, 45.89, 27.98, 25.92, 25.85, -3.76, -4.15, -4.45, -4.58; IR (neat) 3315, 2956, 2930, 2888, 2858, 1749, 1624, 1508 cm-1; m/z 369 (M+H)+.
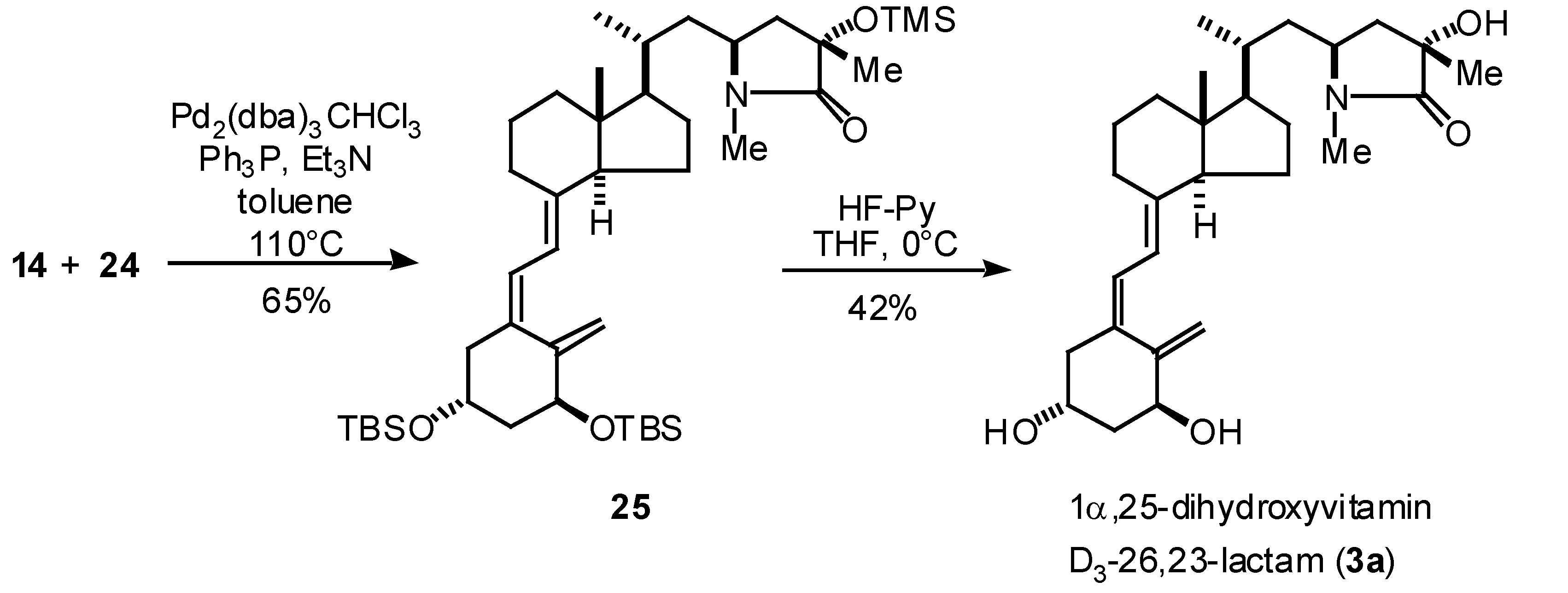
(23S,25S)-1α,3-Di[(t-butyldimethylsilyl)oxyl]-25-(trimethylsilyl)oxyl vitamin D3 26,23-N-methyl lactam (25). A mixture of Pd2(dba)3CHCl3 (2 mg, 0.002 mmol), PPh3 (5 mg, 0.19 mmol) and Et3N (0.8 mL) in toluene (0.8 mL) was stirred for 10 min at room temperature. To the resulting mixture was added 14 (13.8 mg, 0.03 mmol) and 24 (8 mg, 0.02 mmol) in toluene (0.8 mL) dropwise, and the whole mixture was heated at 110°C for 4 h. The reaction mixture was diluted with hexane and concentrated in vacuo. The residue was purified by silica gel column chromatography (hexane) to give 25 (9.4 mg, 62%) as a colorless oil. Ketone 14 (4.7 mg, 33%) and enyne 24 were recovered (2.7 mg, 37%).
(23S,25S)-1α,25-Dihydroxy vitamin D3-26,23-N-methyl lactam (3a). A mixture of 25 (4.7 mg, 0.0062 mmol) and HF pyridine (1 mL) in THF (2 mL) at 0°C was stirred for 6 h. The reaction mixture was quenched with saturated NaHCO3 and the organics were extracted with ethyl acetate. The extracts were dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (9:1 CHCl3-MeOH) to give 3a as a pale yellow oil (1.5 mg, 53%). 1H-NMR (CDCl3) δ 6.37 (d, J = 11.5 Hz, 1H), 6.02 (d, J = 11.1 Hz, 1H), 5.33 (s, 1H), 4.99 (s, 1H), 4.43 (br s, 1H), 4.23 (br s, 1H), 2.82 (s, 3H), 2.61-0.70 (m, 33H), 0.99 (d, J = 6.4 Hz, 3H), 0.58 (s, 3H); m/z 458(M+H)+.