Experimental
General
Thin-layer chromatography (TLC) was accomplished on 0.2-mm precoated plates of silica gel 60 F-254 (Merck). Visualization was made with ultraviolet light (254 and 365 nm) or with a fluorescence indicator. For preparative column chromatography, silica gel 60F 254 Merck (230-240 Mesh ASTM) was used. Melting points were determined on a Kofler melting point apparatus and are uncorrected. 1H‑ NMR spectra were recorded on a Bruker AC 300 P (300 MHz) spectrometer, 13C-NMR spectra on a Bruker AC 300 P (75 MHz) spectrometer. Chemical shifts are expressed in parts per million downfield from tetramethylsilane as an internal standard. Unless otherwise stated, δ values refer to singlet absorptions. Data are given in the following order: δ value, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad), number of protons, coupling constants J are given in Hertz. The mass spectra (HRMS) were taken on a Varian MAT 311 at a ionizing potential of 70 eV in the Centre Régional de Mesures Physiques de l’Ouest (CRMPO, Rennes). Solvents were evaporated with a Buchi rotary evaporator. All reagents were purchased from Acros and Aldrich and were used without purification.
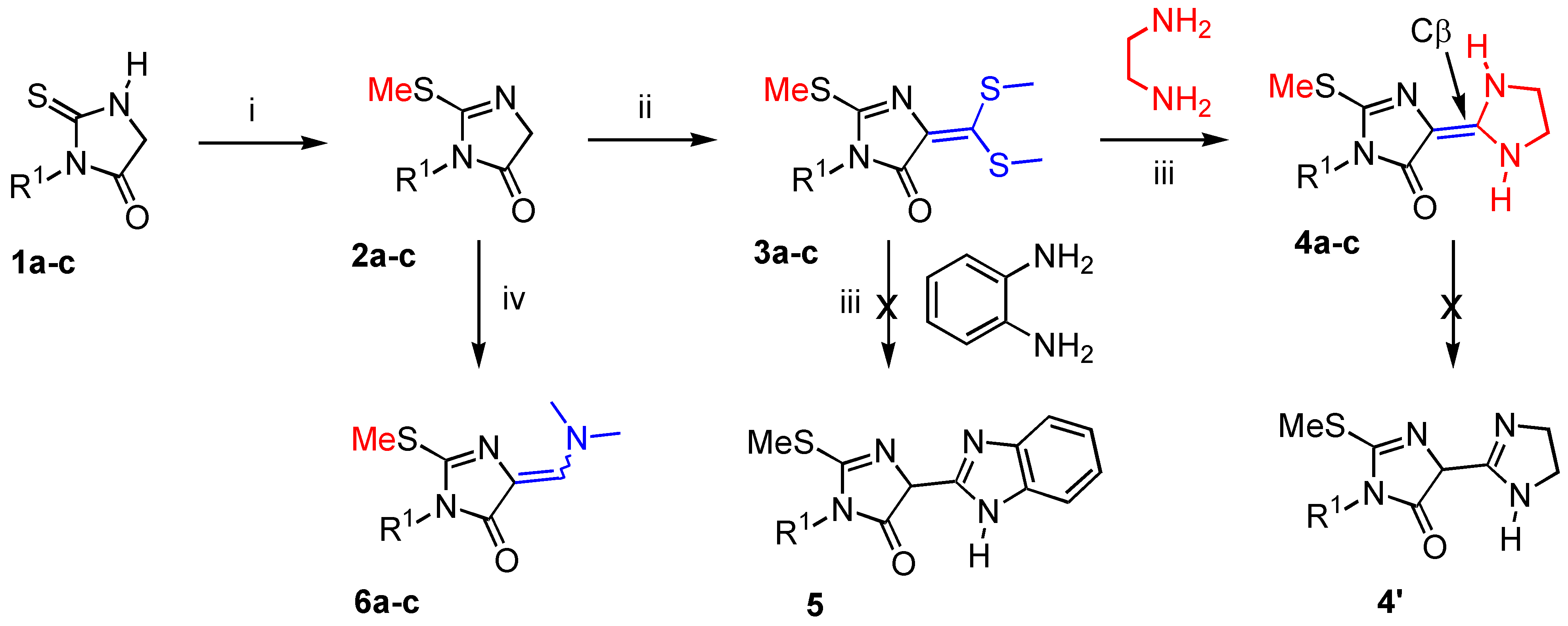
General procedure for the preparation of 2-methylsulfanyl-3,5-dihydro-imidazol-4-ones 2a-c.
To a suspension of thiohydantoin (1, 20 mmol.) and potassium carbonate (1.38 g., 10 mmol.) in anhydrous acetonitrile (50 mL) was added dropwise over 30 minutes methyliodide (4.26 g., 30 mmol.). The reaction mixture was heated in a thermostated oil bath at 40 °C during 14 hours with vigorous magnetic stirring. The reaction mixture was allowed to cool down to room temperature. After elimination of solvent in vacuo, the crude reaction mixture was triturated with diethyl ether (40 mL). After standing for 1 hour, the insoluble salt (KI) was filtered off and washed with diethyl ether (10 mL). The combined filtrates were dried over MgSO4, filtered and elimination of the solvent by rotary evaporation afforded the desired 2-methylsulfanyl-3,5-dihydro-imidazol-4-one 2. The crude products 2a and 2c were purified by recrystallization from pentane and 2b by flash chromatography on silica gel 60F 254 (Merck) with the appropriate eluent.
3-Methyl-2-methylsulfanyl-3,5-dihydroimidazol-4-one (2a). Yield = 95 %; mp = 80-82 °C (from pentane); 1H-NMR (CDCl3) δ: 2.56 (s, 3H), 3.06 (s, 3H), 4.14 (s, 2H); 13C-NMR (CDCl3) δ: 12.3 (q, J = 143 Hz). 26.2 (q, J = 141 Hz), 59.0 (t, J = 144 Hz, C-5), 164.2 (m, C-2, C=N), 180.0 (m, C-4, C=O); HRMS (m /z): found 144.0360 (calc. for C5H8N2OS, M+ requires: 144.0357).
3-Butyl-2-methylsulfanyl-3,5-dihydroimidazol-4-one (2b). Yield = 96 %; viscous oil; Rf = 0.6 (CH2Cl2); 1H-NMR (CDCl3) δ: 0.94 (t, 3H, J= 7,3 Hz), 1.23-1.40 (m, 2H), 1.55-1.65 (m, 2H), 2.55 (s, 3H), 3.47 (t, 2H, J = 7.4 Hz), 4.12 (s, 2H); 13C-NMR (CDCl3) δ: 12.4 (q, J = 143 Hz), 13.5 (qt, J = 125, 3.8 Hz), 19.9 (tm, J = 121 Hz), 30.7 (tm, J = 125 Hz), 40.3 (tm, J = 139, 4.5 Hz), 59.0 (t, J = 144 Hz, C-5), 164.0 (m, C-2, C=N), 179.9 (m, C-4, C=O); HRMS (m /z): found 186.0833 (calc. for C8H14N2OS, M+ requires: 186.0827).
2-Methylsulfanyl-3-phenyl-3,5-dihydroimidazol-4-one (2c). Yield = 90 %; mp = 84-86 °C (from pentane); 1H-NMR (CDCl3) δ: 2.49 (s, 3H), 4.34 (s, 2H), 7.26-7.29 (m, 2H, Ar), 7.39-7.50 (m, 3H, Ar); 13C-NMR (CDCl3) δ: 12.8 (q, J = 143 Hz), 59.4 (t, J = 144 Hz), 127.4 (dt, J = 162, 6.2 Hz), 129.3 (dt, J = 162, 7.5 Hz), 129.5 (dm, J = 163 Hz), 132.1 (t, J = 7.8 Hz), 163.9 (m, C-2, C=N), 179.0 (t, J = 3.5 Hz, C-4, C=O); HRMS (m /z): found 206.0498 (calc. for C10H10N2OS, M+ requires: 206.0514).
General procedure for the preparation of ketene dithioacetals 3a-c.
A mixture of 2-methylsulfanyl-3,5-dihydro-imidazol-4-one 2 (20 mmol.) and carbon disulfide (1.68 g., 22 mmol.) in acetonitrile (50 mL) was treated with anhydrous potassium carbonate (5.53 g., 40 mmol.) under vigorous magnetic stirring at room temperature. Methyl iodide (8.52 g., 60 mmol.) was added dropwise during 45 minutes to the dianionic ambident compound formed. The reaction mixture was stirred for 16 hours at 40 °C. The solvent was removed in vacuo and diethyl ether (100 mL) was poured into the crude reaction mixture. Insoluble salts were filtered off and the filtrate was dried over MgSO4. The solvent was eliminated in a rotary evaporator and the crude reaction mixture gave oil that crystallized on standing. Compounds 3(a-c) were used without further purification.
5-(Bis-methylsulfanyl-methylene)-3-methyl-2-methylsulfanyl-3,5-dihydro-imidazol-4-one (3a). Yield = 60 %; mp = 60-62 °C (from ether); 1H-NMR (CDCl3) δ: 2.58 (s, 3H), 2.63 (s, 3H), 2.73 (s, 3H), 3.14 (s, 3H); 13C-NMR (CDCl3) δ: 12.7 (q, J = 143 Hz), 18.4 (q, J = 141 Hz), 19.2 (q, J = 142 Hz), 26.5 (q, J = 140 Hz), 135.4 (m, C-6), 149.0 (m, C-5), 157.4 (m, C-2, C=N), 163.9 (m, C-4, C=O); HRMS (m /z): found 248.0115 (calc. for C8H12N2OS3, M+ requires: 248.0112).
5-(Bis-methylsulfanyl-methylene)-3-butyl-2-methylsulfanyl-3,5-dihydro-imidazol-4-one (3b). Yield = 68 %; mp = 45-48 °C; 1H-NMR (CDCl3) δ: 0.95 (t, 3H, J = 7.2 Hz), 1.34 (sext, 2H, J = 7.6 Hz), 1.64 (quint, 2H, J = 7.4 Hz), 2.58 (s, 3H), 2.64 (s, 3H); 2.75 (s, 3H), 3.58 (t, 2H, J = 7.3 Hz); 13C-NMR (CDCl3) δ: 12.3 (q, J = 143 Hz), 13.7 (qt, J = 125, 3.8 Hz), 18.4 (q, J = 141 Hz), 19.2 (q, J = 142 Hz), 19.9 (tm, J = 121 Hz), 30.7 (tm, J = 125 Hz), 40.3 (tm, J = 139, 4.5 Hz), 135.4 (m, C=), 149.0 (m, C‑5), 160.1 (m, C-2, C=N), 165.9 (m, C-4, C=O); HRMS (m /z): found 290.0589 (cal. for C11H19N2OS3, M+ requires: 290.0581).
5-(Bis-methylsulfanyl-methylene)-2-methylsulfanyl-3-phenyl-3,5-dihydro-imidazol-4-one (3c). Yield = 70 %; mp = 90-92 °C (from ether); 1H-NMR (CDCl3) δ: 2.58 (s, 3H), 2.59 (s, 3H), 2.79 (s, 3H), 7.27-7.33 (m, 5H, Ar), 7.38-7.50 (m, 3H, Ar); 13C-NMR (CDCl3) δ: 13.2 (q, J = 143 Hz), 18.5 (q, J = 142 Hz), 19.4 (q, J = 142 Hz), 127.2 (ddd, J = 164, 7.3, 5.5 Hz, C-3’), 128.9 (dt, J = 162, 7.5 Hz, C-4’), 129.4 (dd, J = 162, 7.3 Hz, C-2’), 132.8 (t, 9 Hz, C-1’), 134.8 (s); 150.2 (hp, J = 4.8 Hz, C-5); 156,7 (q, J = 5 Hz, C-2, C=N), 163.1 (s, C-4, C=O); HRMS (m /z): found 310.0257 (calc. for C13H14N2OS3, M+ requires: 310.0268).
General procedure for the preparation of 5-(imidazolidin-2-ylidene)-2-methylsulfanyl-3,5-dihydro-imidazol-4-ones 4a-c.
A mixture of ketene dithioacetal (3, 2.4 mmol.), chloroform (10 mL) and freshly distilled ethylene diamine (0.14 g., 1.2 mmol.) was heated with vigorous magnetic stirring in a thermostated oil bath at 70 °C for 8 days (the reaction was monitored by TLC with methylene chloride as eluent). Volatile components were evaporated in vacuo, acetone (10 mL) was added to the residue, and the resulting precipitate was collected by filtration and washed with acetone (1 mL). The product was dried over CaCl2 to give the expected compound as yellowish needles. It is recommended to handle it in the dark under an inert atmosphere at 4 °C.
5-(Imidazolidin-2-ylidene)-3-methyl-2-methylsulfanyl-3,5-dihydro-imidazol-4-one (4a): Yield = 41 %, mp = 124-126 °C (from acetone); 1H-NMR (DMSO-d6) 2.48 (s, 3H), 3.54 (m, 2H), 3.58 (m, 2H), 7.60 (br s, 1H, NH), 7.90 (br s, 1H, NH); 13C-NMR (DMSO-d6, TMS) δ: 13.4 (q, J = 141 Hz), 42.6 (t, J = 145 Hz), 43.7 (t, J = 144 Hz), 100.7 (s, C-5, C=), 134.5 (q, J = 4.8 Hz, C-2, C=N), 163.8 (s, Cβ, C=), 179.8 (s, C-4, C=O); HRMS, (m/z): found 212.0739 (calc. for C9H12N4OS requires 212.0732).
3-Butyl-5-(imidazolidin-2-ylidene)-2-methylsulfanyl-3,5-dihydro-imidazol-4-one (4b): Yield = 43 %, mp = 98-100 °C (from acetone); 1H-NMR (DMSO-d6) δ: 0.91 (t, J = 7.2 Hz), 1.34 (sext, 2H, J = 7.6 Hz), 1.64 (quint, 2H, J = 7.4 Hz), 2.58 (s, 3H), 3.50 (m, 2H), 3.56 (m, 2H), 3.60 (t, 2H, J = 7.3 Hz), 7.64 (br s, 1H, NH), 7.93 (br s, 1H, NH); 13C-NMR (DMSO-d6, TMS) δ: 2.4 (q, J = 143 Hz), 13.5 (qt, J = 125, 3.8 Hz), 19.9 (tm, J = 121 Hz), 30.7 (tm, J = 127 Hz), 40.3 (tm, J = 139, 4.5 Hz), 42.1 (t, J = 145 Hz), 43.9 (t, J = 144 Hz), 101.4 (s, C-5, C=), 140.8 (q, J = 5.2 Hz, C-2, C=N), 164.6 (s, Cβ, C=), 178.9 (s, C-4, C=O); HRMS, (m/z): found 254.1209 (calc. for C11H19N4OS requires 254.1201).
5-(Imidazolidin-2-ylidene)-2-methylsulfanyl-3-phenyl-3,5-dihydro-imidazol-4-one (4c): Yield = 54 %, mp = 228-230 °C (from acetone); 1H-NMR (DMSO-d6) δ: 2.40 (s, 3H), 3.55-3.58 (m, 4H), 7.28-7.50 (m, 5H, Ar), 7.62 (br s, 1H, NH), 7.95 (br s, 1H, NH); 13C-NMR (DMSO-d6, TMS) δ: 13.6 (q, J = 142 Hz), 42.6 (t, J = 144 Hz), 43.8 (t, J = 144 Hz), 100.4 (s, C-5, C=), 127.0 (dm, J = 162 Hz, C-3', Ar), 127.3 (dt, J = 162, 7.5 Hz, C-4', Ar), 128.8 (dd, J = 162, 8 Hz, C-2', Ar), 134.80 (td, J = 9, 1.2 Hz, C-1’, Ar), 135.5 (q, J = 5 Hz, C-2, C=N), 164.2 (s, Cβ, C=), 180.0 (s, C-4, C=O); HRMS, (m/z): found 274.0864 (calc. for C13H14N4OS requires 274.0888).
General procedure for the preparation of 5-dimethylaminomethylene-2-methylsulfanyl-3,5-dihydro-imidazol-4-one 6a-c under microwave irradiation.
A mixture of 2-methylsulfanyl-3,5-dihydro-imidazol-4-one (2, 6.9 mmol.) and commercial dimethylformamide diethylacetal (1.01 g., 6.9 mmol.) was placed in a cylindrical quartz reactor (Ø = 4 cm). The reactor was then introduced into a Synthewave® 402 Prolabo microwave reactor [2.45 GHz, adjusted power within the range 0-300 W and a wave guide (single mode T01) fitted with a stirring device and an IR temperature detector]. The reaction mixture was irradiated at 20% power level (60 W) for 1 hour at 70 °C. Then the mixture was allowed to cool down and a solid formed rapidly (~ 5 min.) at 25 °C. The crude solid was extracted with methylene chloride (20 mL) and the reactor was washed (CH2Cl2 : 10 mL). Volatile components of the combined layers were eliminated in a rotary evaporator under reduced pressure and the crude reaction mixture was purified by flash chromatography on silica gel 60 F 254 (Merck) with the appropriate eluent. Concentration of the desired fraction gave the expected product 6 as yellowish needles.
(5Z) 5-Dimethylaminomethylene-3-methyl-2-methylsulfanyl-3,5-dihydroimidazol-4-one (6a): Yield = 86 %; mp = 134-136 °C; Rf = 0.7 (AcOEt); 1H-NMR (CDCl3) δ: 2.57 (s, 3H), 3.12 (s, 3H), 3.22 (br s, 3H), 3.55 (br s, 3H), 6.95 (s, 1H, =CH); 13C-NMR (CDCl3) δ: 13.1 (q, J = 142 Hz), 26.8 (q, J = 140 Hz), 47.0 (m), 116.0 (s, C-5), 139.1 (d, J = 165 Hz, =CH), 148.5 (m, C-2, C=N), 170.4 (dm, J = 3.4 Hz, C-4, C=O); HRMS, (m/z): found 199.0782 (calc. for C8H13N3OS requires 199.0779).
(5Z) 3-Butyl-5-dimethylaminomethylene-2-methylsulfanyl-3,5-dihydroimidazol-4-one (6b):Yield = 80 %; mp = 80-82 °C; Rf = 0.6 (Et2O); 1H-NMR (CDCl3) δ: 0.86 (t, 3H, J = 7.2 Hz), 1.24 (sext, 2H, J = 7.3 Hz), 1.55 (quint, 2H, J = 7.3 Hz), 2.51 (s, 3H), 3.09 (br s, 3H), 3.50 (br s, 3H), 3.52 (t, 2H, J = 7.1 Hz), 6.87 (s, 1H, =CH). 13C-NMR (CDCl3) δ: 13.4 (q, J = 142 Hz), 14.2 (qt, J = 125, 3.8 Hz), 20.1 (tm, J = 125 Hz), 30.4 (tm, J = 125 Hz), 40.5 (tm, J = 139, 4.5 Hz), 47.6 (m), 116.4 (s, C-5), 139.1 (d, J = 165 Hz, =CH), 149.6 (m, C-2, C=N), 170.1 (dm, J = 3.7 Hz, C-4, C=O); HRMS, (m/z): found 241.1241 (calc. for C11H19N3OS requires 241.1249).
(5Z) 5-Dimethylaminomethylene-2-methylsulfanyl-3-phenyl-3,5-dihydroimidazol-4-one (6c): Yield = 82 %; mp = 138-140 °C; Rf = 0.5 (AcOEt); 1H-NMR (CDCl3) δ: 2.56 (s, 3H), 3.21 (br s, 3H), 3.63 (br s, 3H), 7.07 (s, 1H, =CH), 7.34-7.53 (m, 5H, Ar). 13C-NMR (CDCl3) δ: 13.3 (q, J = 142 Hz), 39,4 (qm, J = 138 Hz), 46.2 (tq, J = 138 Hz), 115.2 (d, J = 5 Hz, C-5), 127.2 (ddd, J = 163, 7.3, 5.4 Hz, Ar), 128.2 (dt, J = 161, 7.5 Hz, Ar), 129.1 (dd, J = 162, 7.9 Hz, Ar), 133.9 (t, J = 9.2 Hz, Ar), 139.1 (dt, J = 166, 3.4 Hz, =CH), 146.7 (t, J = 4.8 Hz, C-2, C=N), 169.1 (d, J = 3.1 Hz, C-4, C=O); HRMS, (m/z): found 261.0924 (calc. for C13H15N3OS requires 261.0936).