Crystal Structure and Characterization of the Dinuclear Cd(II) Complex [Cd(H2O)2(ο-HOC6H4COO)2]2
Abstract
:Introduction
Results and Discussion
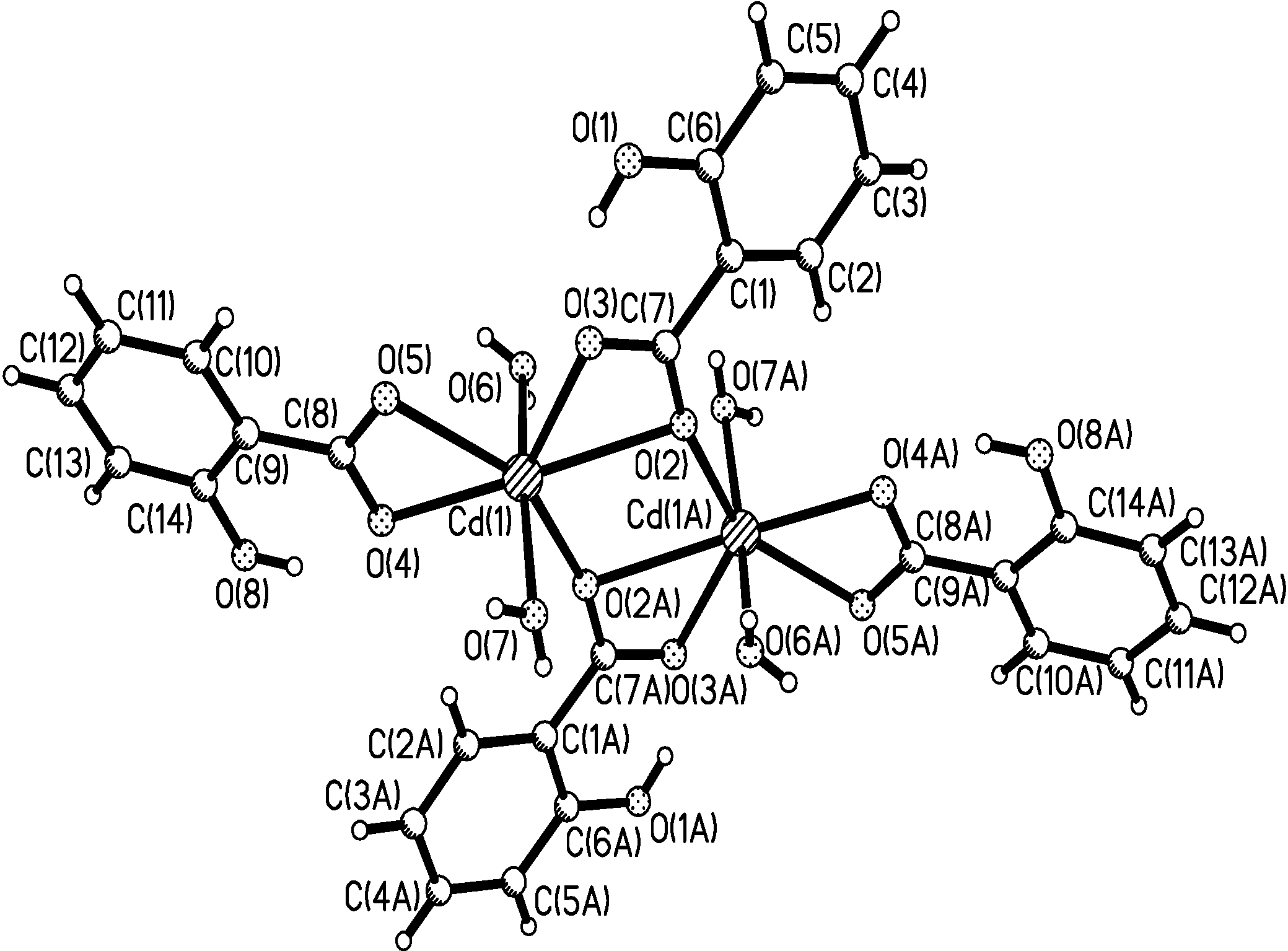
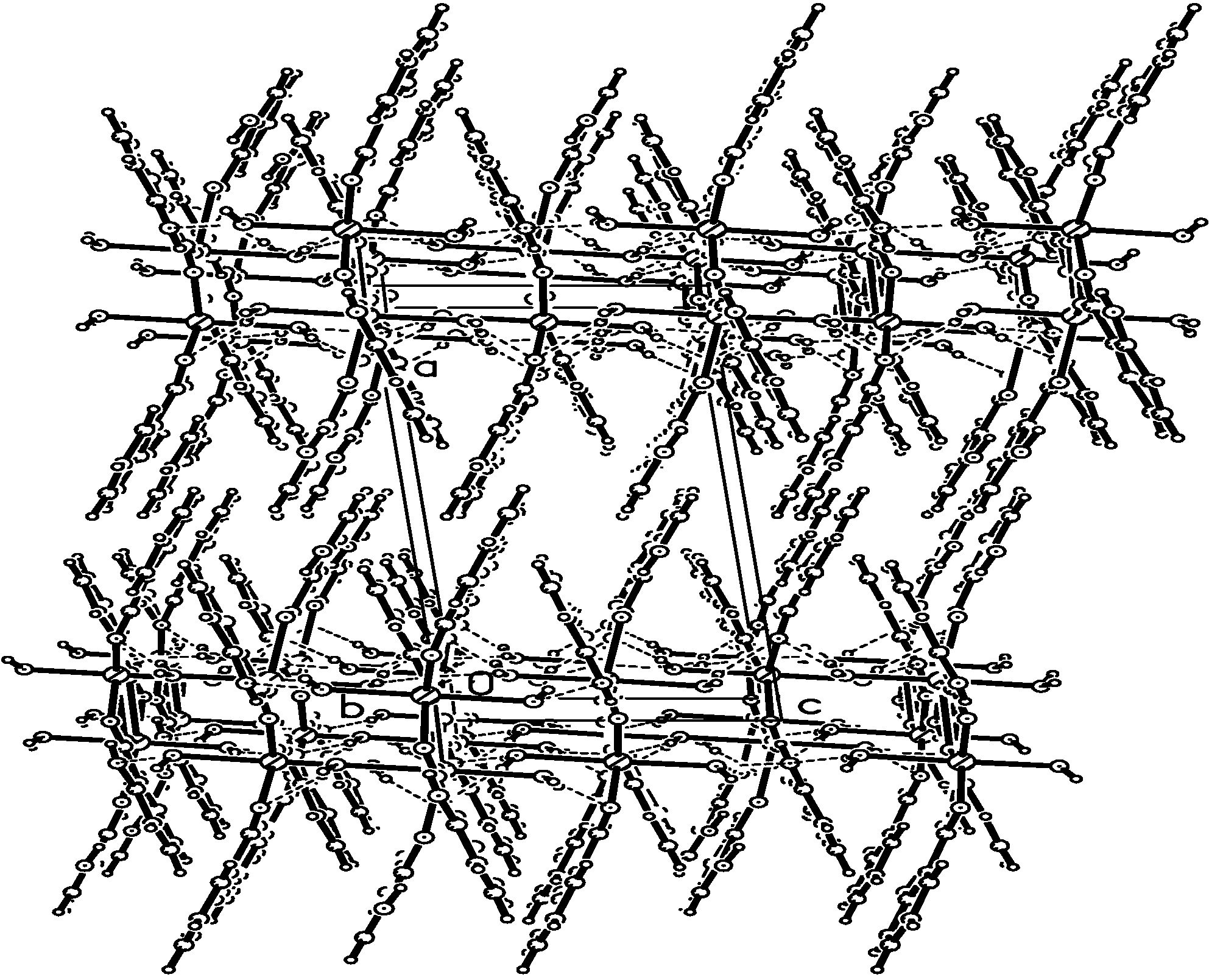
Crystal Structure Study of the Title Compound
| D H A | symm | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|---|
| O(1)-H(1A)···O(3) | – | 0.8200 | 1.7917 | 2.5222 | 147.54 |
| O(8)-H(2B)···O(4) | – | 0.8446 | 1.8319 | 2.5741 | 145.71 |
| O(6)- H(6A)···O(1) | -x,-1/2+y,1/2-z | 0.8200 | 2.0476 | 2.8335 | 160.42 |
| O(6)- H(6B)···O(5) | x,3/2-y,1/2+z | 0.7065 | 2.3152 | 3.0104 | 168.25 |
| O(7)-H(7A)···O(5) | x,3/2-y,-1/2+z | 0.8200 | 1.9050 | 2.7066 | 165.44 |
| O(7)-H(7B)···O(1) | -x,-1/2+y,-1/2-z | 0.7211 | 2.2117 | 2.8687 | 152.05 |
IR Spectra
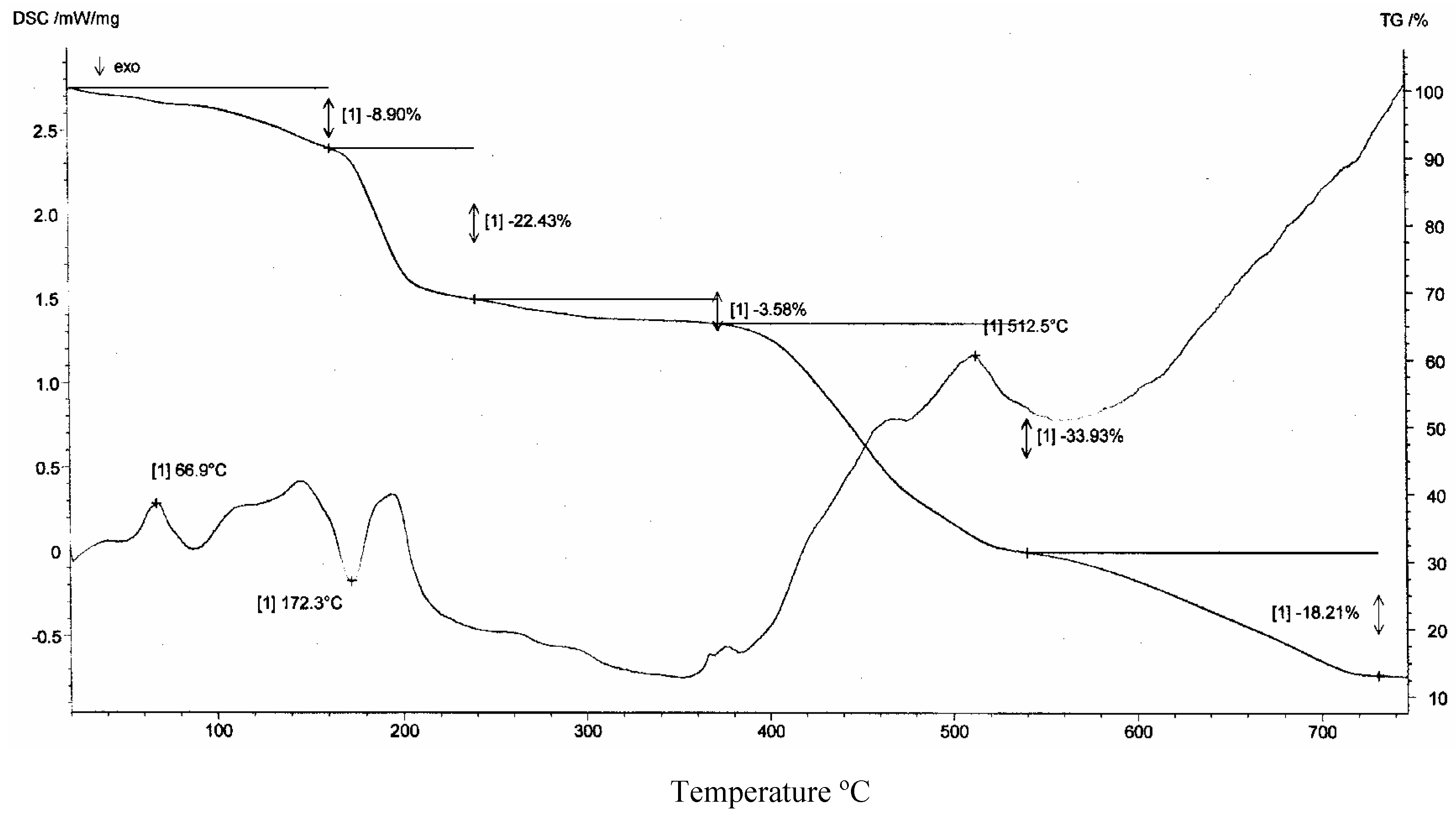
Thermal Analysis
Acknowledgements
Experimental
General
Preparation of [Cd(H2O)2(ο-HOC6H4COO)2]2
X-Ray Diffraction Analysis [15]
| Empirical formula | C14H14CdO8 |
| Formula weight | 422.65 |
| Temperature | 293(2) K |
| Wavelength | 0.71073 Å |
| Crystal system, space group | Monoclinic, P21/c |
| Unit cell dimensions | a = 15.742(3) Å |
| b = 12.451(3) Å β = 96.07(3)o | |
| c = 7.7225(15) Å | |
| Volume | 1505.1(5) Å 3 |
| Z, Calculated density | 4, 1.865 Mg/m3 |
| Absorption coefficient | 1.491 mm-1 |
| F(000) | 840 |
| Crystal size | 0.30 × 0.20 × 0.18 mm |
| Theta range for data collection | 1.30 to 27.58 deg |
| Limiting indices | -20 ≤ h ≤ 20, -16 ≤ k ≤ 16, 0 ≤ l ≤ 10 |
| Reflections collected / unique | 5198 / 3024 [R(int) = 0.0322] |
| Completeness to theta = 27.43 | 86.9 % |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 3024 / 0 / 220 |
| Goodness-of-fit on F2 | 0.986 |
| Final R indices [I>2σ(I)] | R1 = 0.0321, wR2 = 0.0628 |
| R indices (all data) | R1 = 0.0495, wR2 = 0.0658 |
| Largest diff. peak and hole | 0.379 and -1.177 e. Å -3 |
References
- Caroll, P.E. J. Am. Med. Assoc. 1966, 198, 267. [CrossRef]
- Schroeder, H.S.; Balassa, J.J. Am. J. Physiol. 1965, 209, 433.
- Li, X.F.; Dong, J.T.; Wei, H.J.; Luo, X.M. Develop Biol. Chem. Biol. Phy. 1992, 19, 72.
- Yang, J.R.; He, J.Q.; Zhang, G.X.; Mao, X.Q. Chin. Enviro. Sci. 1996, 16, 1000.
- Clegg, W.; Cressey, J.T.; McCamley, A.; Straughan, B.P. Acta Crystallogr. Sect. C 1995, 51, 234.
- Rardin, R.L.; Tolman, W.B.; Lippard, S.J. New J. Chem. 1991, 15, 417.
- Xiong, R.G.; Liu, C.M.; Zuo, J.L.; You, X.Z. Inorg. Chem. Commu. 1999, 2, 292.
- Liu, C.M.; Xiong, R.G.; You, X.Z.; Chen, W.; Lo, K.M. J. Coord. Chem. 1998, 46, 211.
- Xu, X.Y.; Wang, Z.L.; Luo, Q.H.; Shen, M.C.; Zhou, X.G.; Zhou, Z.Y. J. Coord. Chem. 1998, 43, 281. [CrossRef]
- Xiao, W.; Lu, Z.L.; Su, C.Y. J. Mol. Struct. 2000, 533, 91. [CrossRef]
- Hunter, R.H.; Haueisen, R.H.; Irving, A. Angew. Chem. Int. Ed. Engl. 1994, 33, 566.
- Chesnut, D.J.; Kusnetzow, A.; Birge, R.; Zubieta, J. J. Chem. Soc., Dalton Trans. 2001, 2581.
- Jian, F.F.; Wang, Z.X.; Bai, Z.P.; You, X.Z.; Chen, W. Trans. Met. Chem. 1999, 24, 589.
- Jian, F.F.; Wang, Z.X.; Bai, Z.P.; You, X.Z.; Fun, H.K.; Chinnakali, K. J. Chem. Crystallogr. 1999, 24, 589.
- CCDC 241966 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ; fax +44 1223 336033; e-mail: [email protected]).
- Sheldrick, G.M. SADABS. In Program for Empirical Absorption Correction of Area Detector Data; University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXTL V5.1 Software Reference Manual; Bruker AXS, Inc.: Madison, Wisconsin, USA, 1997. [Google Scholar]
- Wilson, A.J.C. International Tables for X-ray Crystallography, Vol. C; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; Tables 6.1.1.4 (pp. 500-502) and 4.2.6.8 (pp. 219-222), respectively. [Google Scholar]
- Sample availability: Available from the authors.
© 2004 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jian, F.; Xiao, H.; Sun, P.; Zhao, P. Crystal Structure and Characterization of the Dinuclear Cd(II) Complex [Cd(H2O)2(ο-HOC6H4COO)2]2. Molecules 2004, 9, 876-882. https://doi.org/10.3390/91000876
Jian F, Xiao H, Sun P, Zhao P. Crystal Structure and Characterization of the Dinuclear Cd(II) Complex [Cd(H2O)2(ο-HOC6H4COO)2]2. Molecules. 2004; 9(10):876-882. https://doi.org/10.3390/91000876
Chicago/Turabian StyleJian, Fangfang, Hailian Xiao, Pingping Sun, and Pusu Zhao. 2004. "Crystal Structure and Characterization of the Dinuclear Cd(II) Complex [Cd(H2O)2(ο-HOC6H4COO)2]2" Molecules 9, no. 10: 876-882. https://doi.org/10.3390/91000876




