Synthesis of Substituted Stilbenes via the Knoevenagel Condensation
Abstract
:Introduction
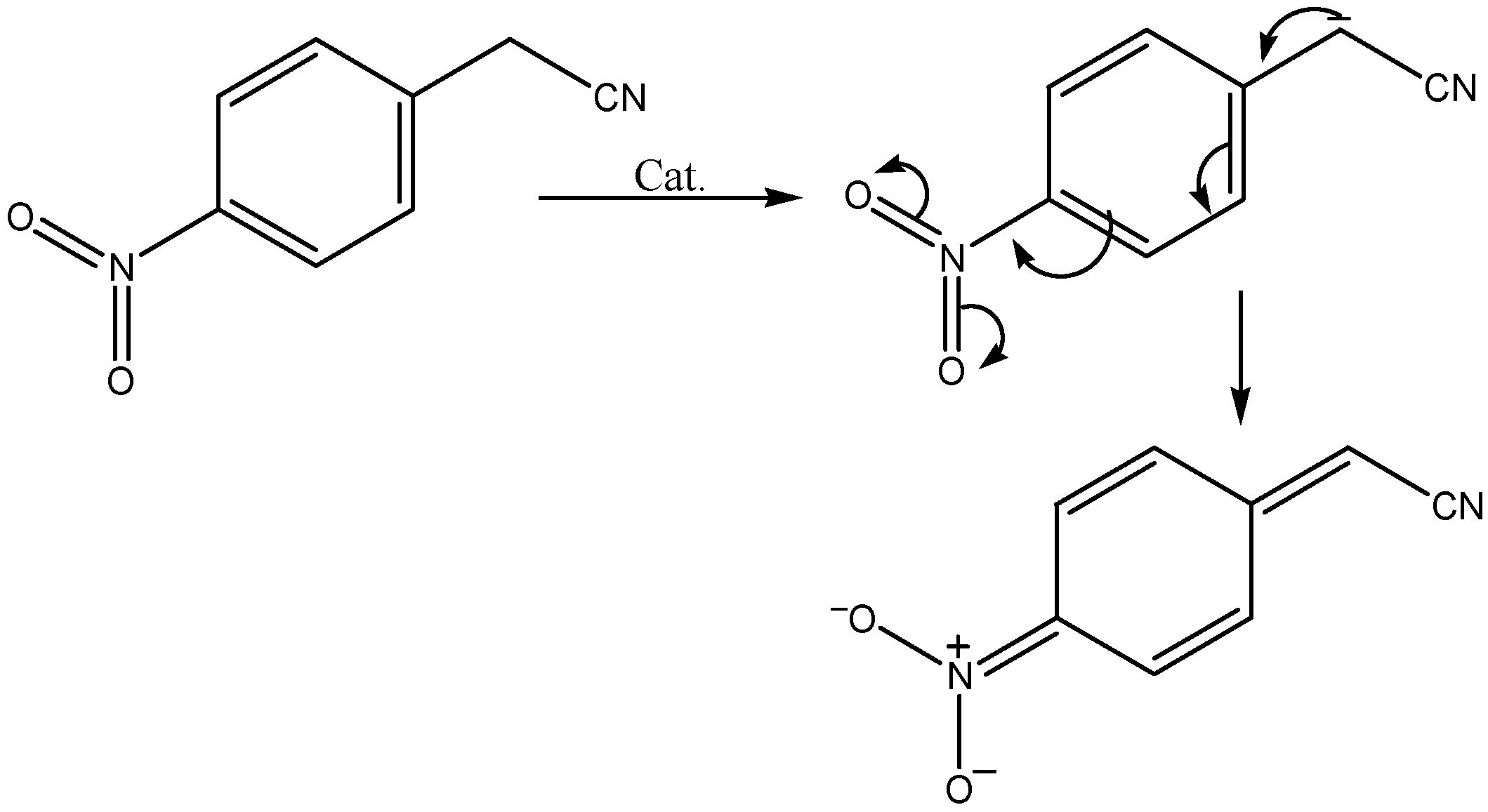
Results and Discussion
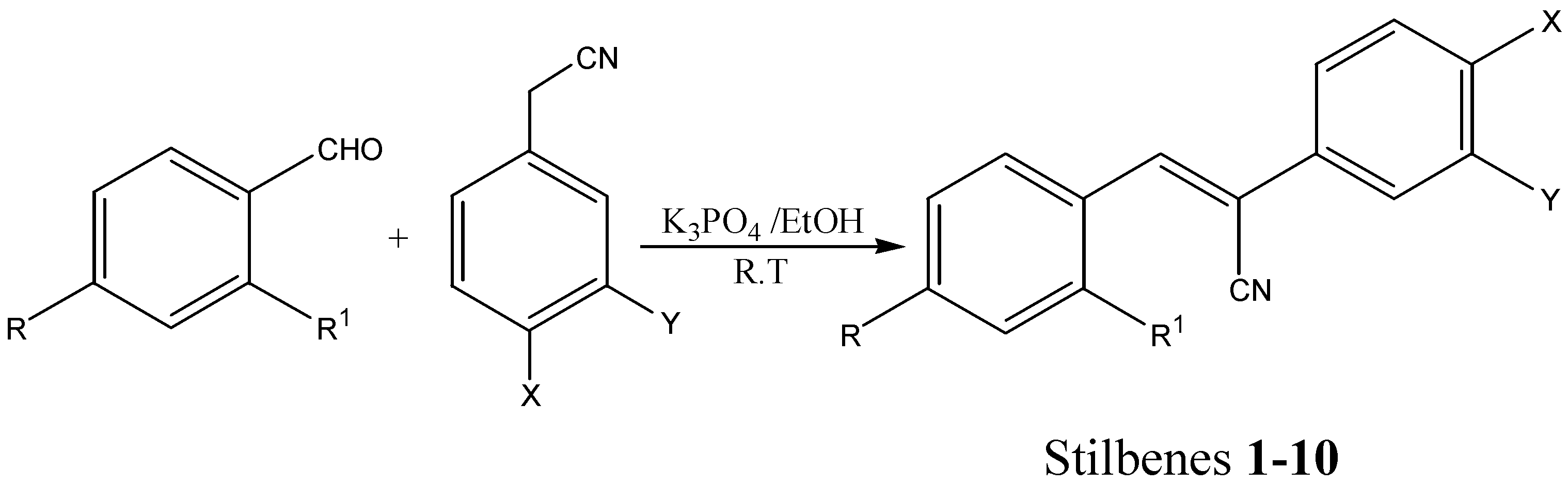
| Stilbene No. | R | R1 | X | Y |
|---|---|---|---|---|
| 1 | H | H | F | H |
| 2 | Br | H | F | H |
| 3 | Cl | H | F | H |
| 4 | NO2 | H | F | H |
| 5 | (CH3)2N | H | F | H |
| 6 | H | OEt | F | H |
| 7 | (CH3)2N | H | Cl | Cl |
| 8 | H | H | NO2 | H |
| 9 | (CH3)2N | H | NO2 | H |
| 10 | Cl | H | NO2 | H |
| Comp. No | Yield (%) | Colour | m. p. (°C) | Molecular Formula | Calculated (Found) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| 1 | 95 | colourless | 88-89 | C15H10FN | 80.70 | 4.51 | 6.27 |
| (79.98) | (4.51) | (6.37) | |||||
| 2 | 91 | colourless | 89-90 | C15H9BrFN | 59.63 | 3.00 | 4.64 |
| (59.65) | (3.01) | (4.72) | |||||
| 3 | 94 | colourless | 104-105 | C15H9ClFN | 69.91 | 3.52 | 5.44 |
| (68.76 ) | (3.57) | (5.48) | |||||
| 4 | 86 | pale yellow | 110-111 | C15H9FN2 | 67.16 | 3.38 | 10.44 |
| (67.45 ) | (3.30) | (10.20) | |||||
| 5 | 92 | yellow | 180-181 | C17H15FN2 | 76.67 | 5.68 | 10.52 |
| (77.20) | (6.10) | (10.78) | |||||
| 6 | 82 | colourless | 95-96 | C17H14FNO | 76.39 | 5.28 | 5.24 |
| (76.03 ) | (5.28) | (5.29) | |||||
| 7 | 91 | yellow | 213-214 | C17H14Cl2N2 | 64.37 | 4.45 | 8.83 |
| (63.10) | (4.21) | (8.77) | |||||
| 8 | 85 | pale yellow | 95-96 | C15H10N2O2 | 71.99 | 4.03 | 11.19 |
| (70.49) | (3.83) | (11.00) | |||||
| 9 | 87 | brown | 253-254 | C17H15N3O2 | 69.61 | 5.15 | 14.33 |
| (68.54) | (5.02) | (14.05) | |||||
| 10 | 87 | yellow | 167-168 | C15H9ClN2O2 | 63.28 | 3.19 | 9.84 |
| (63.10) | (3.09) | (9.79) | |||||
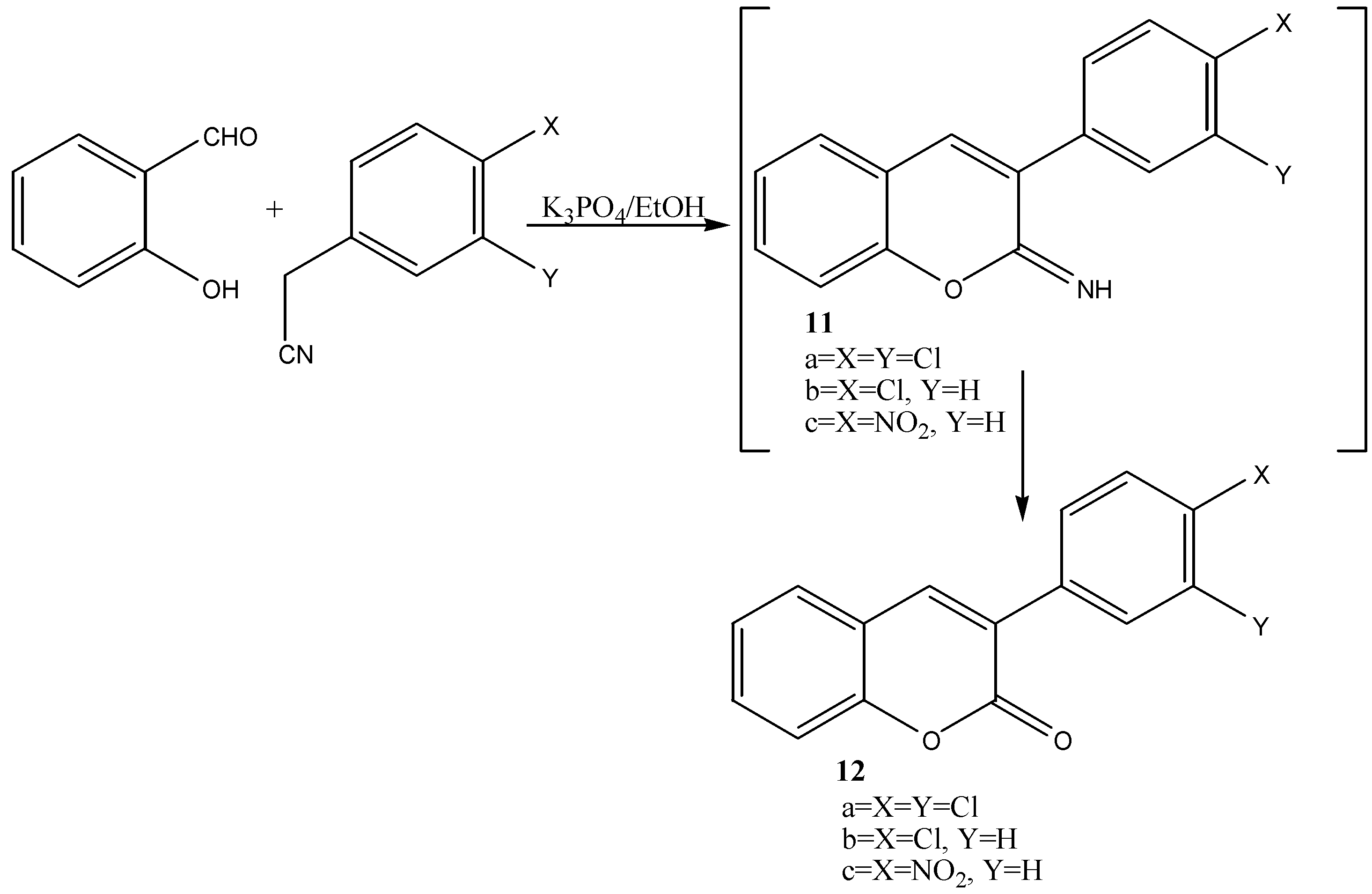
| 12a | 85 | yellow | 191-192 | C15H8Cl2O2 | 61.88 | 2.77 | - |
| (62.07) | ( 2.86) | ||||||
| 12b | 82 | yellow | 136-137 | C15H9ClO2 | 70.19 | 3.53 | - |
| (70.45) | (3.91) | ||||||
| 12c | 75 | pale yellow | 266 | C15H9NO4 | 67.42 | 3.39 | 5.24 |
| (67.03) | (3.31) | (5.76) | |||||
1H-NMR spectra
| Comp. No | δ ppm in (DMSO) | IR/cm-1 | ||
|---|---|---|---|---|
| C≡N | C=C | CH | ||
| 1 | 8.00 (s, 1H), 7.91 (d, J= 9.0 Hz, 2H), 7.80 (m, 2H), 7.55 (m, 3H), 7.38 (t, J= 8.7 Hz, 2H), | 2214 | 1605 | 2955 |
| 2 | 7.99 (s, 1H), 7.87 – 7.76 (m, 7H), 7.33 (t, J= 8.0 Hz, 1H). | 2206 | 1593 | 2924 |
| 3 | 8.01 (s, 1H), 7.92 (2H, d, J= 8.4 Hz), 7.82 (m, 2H), 7.62 (d, J= 8.4 Hz, 2H), 7.36 (t, J= 8.4 Hz, 2H). | 2225 | 1600 | 2940 |
| 4 | 8.40 (d, J= 8.4 Hz, 4H), 8.17 (s, 1H), 8.14 (d, J= 8.4 Hz, 4H). | 2207 | 1593 | 2924 |
| 5 | 7.84 (d, J= 8.9 Hz, 2H), 7.75 (s, 1H), 7.70 (m, 2H), 7.29 (t, J = 8.9 Hz, 2H), 6.80 (d, J = 8.9 Hz, 2H), 3.02 (s, 6H), | |||
| 6 | 7.97 (s, 1H), 7.90 (d, J= 8.8 Hz, 1H), 7.74 (m, 2H), 7.47 (m, 1H), 7.35 (t, J= 8.8 Hz, 2H), 7.14 (d, J= 8.8 Hz, 1H), 7.10 (t, J= 8.8 Hz, 1H), 4.13 (d, J =7.2 Hz, 2H), 1.36 (t, J =7.2 Hz, 3H). | 2212 | 1590 | 2988 |
| 7 | 7.91 (s, 1H), 7.89 (m, 3H), 7.62 (m, 2H), 6.82 (d, J = 9.0 Hz, 2H), 3.06 (s, 6H). | 2207 | 1593 | 2910 |
| 8 | 8.32 (m, 3H), 8.05 (s, 1H), 8.00 (m, 3H), 7.58 (m, 3H). | 2218 | 1589 | 2931 |
| 9 | 8.27 (m, 2H), 8.05 (s, 1H), 7.94 (m, 4H), 6.83 (d, J= 8.8 Hz, 2H), 3.06 (s, 3H). | 2206 | 1585 | 2909 |
| 10 | 8.35 (d, J= 8.7 Hz, 2H), 8.29 (s, 1H), 8.04 (t, J= 8.7, 8.4 Hz, 4H), 7.65 (d, J= 8.4 Hz, 2H). | 2217 | 1593 | 2931 |
| 12a | 8.30 (s, 1H), 7.80-7.60 (m, 3H), 7.61 (t, J= 7.5 Hz, 1H), 7.53 (d, J= 7.5 Hz, 1H), 7.45-7.39 (m, 2H). | |||
| 12b | 8.33 (s, 1H), 7.67 (m, 1H), 7.56-7.45 (m, 4H), 7.19 (m, 2H), 6.96 (m, 1H). | |||
| 12c | 8.47 (br s, 1H), 8.33 (d, J= 8.5 Hz, 2H), 8.03 (d, J= 8.5 Hz), 7.82 (dd, J=8.0, 1.5 Hz), 7.66 (dd, J= 8.5 Hz, 1H), 7.46-7.40 (m, 2H). | |||
IR spectral data
MS spectral data
UV-Vis Spectra
| Comp. No. | Trans λmax (nm) | Cis λmax (nm) | Max irradiation time (h) |
|---|---|---|---|
| 1 | 316 | 311 | 8 |
| 2 | 336 | 327 | 10 |
| 3 | 322 | 315 | 10 |
| 4 | 338 | 323 | 10 |
| 5 | 395 | 358 | 8 |
| 6 | 342 | 329 | 6 |
| 7 | 410 | 396 | 6 |
| 8 | 330 | 324 | 10 |
| 9 | 448 | 433 | 6 |
| 10 | 342 | 322 | 6 |
Experimental
General
General procedure for the Knoevenagel condensations
References
- Jenner, G. Tetrahedron Lett. 2001, 42, 243–245.
- Anselmi, E.; Blazejewsk, J. I.; Wakselman, C. J. Fluorine Chem. 2001, 107, 315–319.
- Trost, B.M. Comprehensive Organic Synthesis; Pergamon Press: Oxford, 1991; vol. 2, pp. 133–340. [Google Scholar]
- March, J. Advanced Organic Chemistry, 4th Ed.; Wiley & Sons: Chichester, New York, 1992; pp. 835–841. [Google Scholar]
- Sanna, P.; Carta, A.; Nikookar, M. E. R. Eur. J. Med. Chem. 2000, 35, 535–543.
- Varma, R. S.; Kabalka, G. W.; Evans, L. T.; Pagni, R. M. Synth. Commun. 1985, 15(4), 279–284.
- Yamawaki, J.; Kawate, A. T. T.; Hanafusa, T. Bull. Chem. Soc., Jpn. 1983, 56, 1885–1892.
- Angeletti, E.; Canepa, C.; Martinetti, G.; Venturello, P. J. Chem. Soc. Perkin Trans 1. 1989, 105–107.
- Texier-Boullet, F.; Foucaud, A. Tetrahedron Lett. 1982, 23, 4927–4928.
- Moison, H.; Texier-Boullet, F.; Foucaud, A. Tetrahedron 1987, 43, 537–542.
- Berrée, F.; Marchad, E.; Morel, G. Tetrahedron Lett. 1992, 33, 6155–6158.
- Stankovic, E.; Toma, S.; Boxel, R.; Asselberghs, I.; Persoons, A. J. Organomet. Chem. 2001, 637-639, 426–433.
- Saito, T.; Goto, H.; Honda, K.; Fujii, T. Tetrahedron Lett. 1992, 33, 7535–7538.
- Sebti, S.; Saber, A.; Rhihil, A.; Nazih, R. Appl. Catal. A 2001, 206, 217–220.
- Aramendia, M. A.; Borau, V.; Jimenez, C.; Marinas, J. M.; Romero, F. J. React. Kinet. Catal. Lett. 2000, 69, 311–316.
- Sebti, S.; Solhy, A.; Tahir, R.; Abdelatif, S.; Boulaajaj, A.; Mayoral, J.; Garcia, J.; Fraile, J.; Kossir, A.; Oumimoun, H. J. Catal. 2003, 213, 1–6.
- Li, Y-Q. J. Chem. Research (S) 2000, 524–525.
- Waldeck, D. H. Chem. Rev. 1991, 91, 415–436.
- Rodriguez, I.; Iborra, S.; Rey, F.; Corma, A. Applied Catalysis A: General 2000, 194-195, 241–252.
- Rao, P. S.; Venkataratnam, R. V. Tetrahedron Lett. 1991, 32, 5821–5822.
- Kvaran, Á.; Ásgeirsson, B. I.; Geirsson, J. K. F. J. Mol. Struct. 2001, 563-564, 513–516.
- Sample availability: Contact the author.
© 2004 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Al-Shihry, S.S. Synthesis of Substituted Stilbenes via the Knoevenagel Condensation. Molecules 2004, 9, 658-665. https://doi.org/10.3390/90800658
Al-Shihry SS. Synthesis of Substituted Stilbenes via the Knoevenagel Condensation. Molecules. 2004; 9(8):658-665. https://doi.org/10.3390/90800658
Chicago/Turabian StyleAl-Shihry, Shar Saad. 2004. "Synthesis of Substituted Stilbenes via the Knoevenagel Condensation" Molecules 9, no. 8: 658-665. https://doi.org/10.3390/90800658





