Synthesis and Evaluation of a Molecularly Imprinted Polymer for 2,4-Dinitrophenol
Abstract
:1. Introduction
2. Results and Discussion
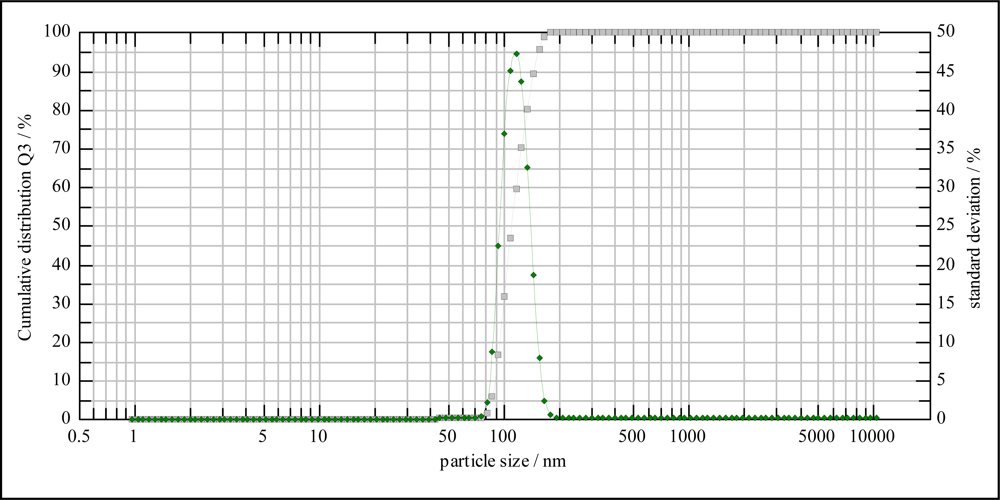
2.1. Characterization of MIP
2.2. pH Study
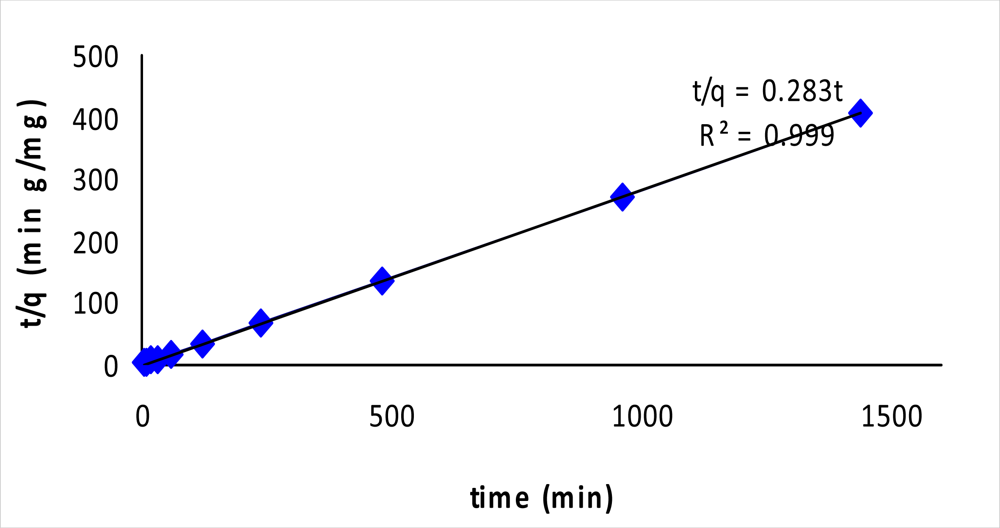
2.3. Kinetic Study
2.4. Adsorption Isotherm Study
2.5. Selectivity Study
3. Experimental Section
3.1. Materials
3.2. Preparation of 2,4-dinitrophenol-imprinted Polymer
3.3. Adsorption Studies
3.4. Characterization of MIP
4. Conclusions
Acknowledgments
References and Notes
- Puig, D; Barcelo, D. Determination of polar priority phenols at parts per trillion levels in water using on-line liquid-solid extraction followed by liquid chromatography with coulimetric detection. J. Chromatogr. A 1997, 778, 313–319. [Google Scholar]
- Tomei, MC; Rossetti, S; Annesini, MC. Microbial and kinetic characterization of pure and mixed cultures aerobically degarding 4-nitrophenol. Chemosphere 2006, 63, 1801–1808. [Google Scholar]
- Wu, Z; Cong, Y; Zhou, M; Ye, Q; Tan, T. Removal of phenolic compounds by electroassisted advanced process for wastewater purification. Kor J. Chem. Eng 2002, 19, 866–870. [Google Scholar]
- Zaggout, FR; Ghalwa, NA. Removal of o-nitrophenol from water by electrochemical degradation using a lead oxide/titanium modified electrode. J. Environ. Manage 2008, 86, 291–296. [Google Scholar]
- Belaid, C; Kallel, M; Khadhraou, M; Lalleve, G; Elleuch, B; Fauvarque, JF. Electrochemical treatment of olive mill wastewaters: Removal of phenolic compounds and decolourization. J. Appl. Electrochem 2006, 36, 1175–1182. [Google Scholar]
- Ku, Y; Lee, KC. Removal of phenols from aqueous solution by XAD-4 resin. J. Hazard. Mater 2007, 80, 59–68. [Google Scholar]
- Oh, CG; Ahn, JH; Ihm, SK. Adsorptive removal of phenolic compounds by using hypercrosslinked polystyrenic beads with bimodal pore size distribution. Sep. Purif. Technol 2004, 38, 173–179. [Google Scholar]
- El-Hamshary, H; El-Sigeny, S; Manal, F; Taleb, A; El-Kelesh, NA. Removal of phenolic compounds using (2-hydroxyethylmethacrylate/acrylamidopyridine) hydrogel prepared by gamma radiation. Sep. Purif. Technol 2007, 57, 329–337. [Google Scholar]
- Kadhim, H; Graham, C; Barrat, P; Evans, CS; Rastall, RA. Removal of phenolic compounds in water using Coriolus versicolor grown on wheat bran. Enzyme Microb. Technol 1999, 24, 303–307. [Google Scholar]
- Damaris, NM; Shiundu, PM; Ndonye, RM; Kamau, GN. Adsorption and detection of some phenolic compounds by rice husk ash of Kenyan origin. Environ. Monit 2002, 4, 978–984. [Google Scholar]
- Caro, E; Marcé, RM; Cormack, PG; Sherrington, DC; Borull, F. On-line solid-phase extraction with molecularly imprinted polymers to selectively extract substituted 4-chlorophenols and 4-nitrophenol from water. J. Chromatogr. A 2003, 995, 233–238. [Google Scholar]
- Ricardo, C; Tarley, T; Kubota, LT. Molecularly-imprinted solid phase extraction of catechol from aqueous effluents for its selective determination by differential pulse voltammetry. Anal. Chim. Acta. 2005, 548, 11–19. [Google Scholar]
- Guney, O; Yilmaz, Y; Pekcan, O. Metal ion templated chemosensor for metal ions based on flouresence quenching. Sens. Actuat. B 2002, 85, 86–89. [Google Scholar]
- Kataky, R; Morgan, E. Potential of enzyme mimics in biomimetic sensors: A modified cyclodextrin as a dehydrogenase enzyme mimic. Biosens. Bioelectron 2003, 18, 1407–1417. [Google Scholar]
- Weiss, R; Molinelli, A; Jakusch, M; Mizaikoff, B. Molecular imprinting and solid phase extraction of flavonoid compounds. Bioseparation 2001, 10, 379–387. [Google Scholar]
- Walshe, M; Garcia, E; Howarth, JM; Smyth, R; Kelly, MT. Separation of the enantiomers of propranolol by incorporation of molecularly imprinted polymer particles as chiral selectors in capillary electrophoresis. Anal. Commun 1997, 34, 119–122. [Google Scholar]
- Yang, G; Liu, H; Wang, M; Liu, S; Chen, Y. Chromatographic characterization and solid-phase extraction on diniconazole-imprinted polymers stationary phase. React. Funct. Polym 2006, 66, 579–583. [Google Scholar]
- Zhang, H; Song, T; Zong, F; Chen, T; Pan, C. Synthesis and characterization of molecularly imprinted polymers for phenoxyacetic acids. Int. J. Mol. Sci 2008, 9, 998–106. [Google Scholar]
- Shim, YH; Yilmaz, E; Lavielle, S; Haupt, K. Chiral recognition and separation of β2-amino acids using non-covalently molecularly imprinted polymers. Analyst 2004, 129, 1211–1215. [Google Scholar]
- Adil, ED; Sener, I. Removal of phenolic compound with nitrophenol-imprinted polymer based on π-π and hydrogen-bonding interactions. Sep. Purif. Technol 2004, 38, 173–179. [Google Scholar]







| Kd (MIP) (mg/g) | Kd (NIP) (mg/g) | k (MIP) | k(NIP) | k’ | |
|---|---|---|---|---|---|
| 2,4-Dinitrophenol | 3.8 | 1.7 | |||
| Phenol | 1.5 | 1.9 | 2.5 | 0.88 | 2.88 |
| 3-Chlorophenol | 1.5 | 1.7 | 2.4 | 0.99 | 2.46 |
| 2,4-Dichlorophenol | 2.2 | 1.9 | 1.7 | 0.89 | 1.95 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zakaria, N.D.; Yusof, N.A.; Haron, J.; Abdullah, A.H. Synthesis and Evaluation of a Molecularly Imprinted Polymer for 2,4-Dinitrophenol. Int. J. Mol. Sci. 2009, 10, 354-365. https://doi.org/10.3390/ijms10010354
Zakaria ND, Yusof NA, Haron J, Abdullah AH. Synthesis and Evaluation of a Molecularly Imprinted Polymer for 2,4-Dinitrophenol. International Journal of Molecular Sciences. 2009; 10(1):354-365. https://doi.org/10.3390/ijms10010354
Chicago/Turabian StyleZakaria, Nor Dyana, Nor Azah Yusof, Jelas Haron, and Abdul Halim Abdullah. 2009. "Synthesis and Evaluation of a Molecularly Imprinted Polymer for 2,4-Dinitrophenol" International Journal of Molecular Sciences 10, no. 1: 354-365. https://doi.org/10.3390/ijms10010354




