Biodegradation of Poly(butylene succinate) Powder in a Controlled Compost at 58 °C Evaluated by Naturally-Occurring Carbon 14 Amounts in Evolved CO2 Based on the ISO 14855-2 Method
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
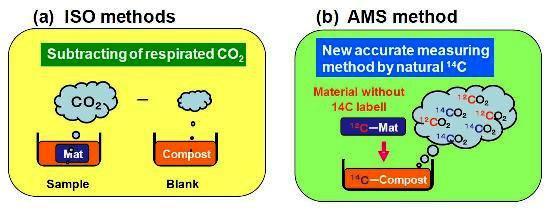
2.2. Biodegradation Test by MODA Apparatus Based on ISO 14855-2 (ISO Method)
2.3. Biodegradation Test by MODA Apparatus with Carbon Dioxide Trap Including the Ba(OH)2 Aqueous Solution
2.4. Graphite Preparation from BaCO3 for AMS Measurements
2.5. Measurement of Percent Modern Carbon
3. Results and Discussion
3.1. Biodegradation of PBS Evaluated from the Blank and Sample Vessels Based on ISO 14855-2
3.2. Biodegradability Determined in One Compost Vessel without a Blank Vessel Using Percent Modern Carbon Determined by AMS
4. Conclusions
References
- Michel, V. Aliphatic polyesters: Great degradable polymers that cannot do everything. Biomacromolecules 2005, 6, 538–546. [Google Scholar]
- .
- Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 2: Gravimetric Measurement of Carbon Dioxide Evolved In a Laboratory-Scale Test; ISO 14855-2; ISO: Geneva, Switzerland, 2007.
- Karlsson, S; Ljungquist, O; Albertsson, A-C. Biodegradation of polyethylene and the influence of surfactants. Polym. Degrad. Stab 1988, 21, 237–250. [Google Scholar]
- Standard Test Methods for Determining Aerobic Biodegradation of Radiolabeled Plastic Materials in an Aqueous or Compost Environment; ASTM D6340–98ASTM: West Conshohocken, PA, USA, 1998.
- Standard Test Method for Determining the Biodegradability of Radiolabeled Polymeric Plastic Materials in Seawater; ; ASTM D6692–01ASTM: West Conshohocken, PA, USA, 2001.
- Standard Test Method for Determining Anaerobic Biodegradability of Radiolabeled Plastic Materials in a Laboratory-Scale Simulated Landfill Environment; ; ASTM D6776–02ASTM: West Conshohocken, PA, USA, 2002.
- Federie, TW; Bariaz, MA; Pettigrew, CA; Kerr, KM; Kemper, JJ; Nuck, BA; Schechtman, LA. Anaerobic biodegradation of aliphatic polyesters: Poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) and poly(e-caprolactone). Biomacromolecules 2002, 3, 813–822. [Google Scholar]
- Ponsart, S; Coudane, J; Saulnier, B; Morgat, J-L; Vert, M. Biodegradation of [3H] poly(ɛ-caprolactone) in the presence of active sludge extracts. Biomacromolecules 2001, 2, 373–377. [Google Scholar]
- Vert, M; Santos, ID; Ponsart, S; Alauzet, N; Morgat, J-L; Coudane, J. Degradable polymers in a living environment: Where do you end up? Polym. Int 2002, 51, 840–844. [Google Scholar]
- Kunioka, M; Ninomiya, F; Funabashi, M. Novel evaluation method of biodegradabilities for oil-based polycaprolactone by naturally occurring radiocarbon-14 concentration using accelerator mass spectrometry based on ISO 14855-2 in controlled compost. Polym. Degrad. Stab 2007, 92, 1279–1288. [Google Scholar]
- Standard Test Methods for Determining the Biobased Content of Solid, Liquid, and Gaseous Samples using Radiocarbon Analysis; ; ASTM D6866–08ASTM: West Conshohocken, PA, USA, 2008.
- Narayan, R. Biobased and Biodegradable Polymer Materials; ACS Polymer Preprints: San Diego, CA, USA, 2005; pp. 319–320. [Google Scholar]
- Currie, LA; Klinedinst, DB; Burch, R; Feltham, N; Dorsch, R. Authentication and dating of biomass components of industrial materials; links to sustainable technology. Nucl. Instrum. Methods Phys.Res. Sect. B 2000, 172, 281–287. [Google Scholar]
- Kunioka, M; Inuzuka, Y; Ninomiya, F; Funabashi, M. Biobased contents of biodegradable poly(ɛ-caprolactone) composites polymerized and directly molded using aluminium triflate from caprolactone with cellulose and inorganic filler. Macromol. Biosci 2006, 6, 517–523. [Google Scholar]
- Kunioka, M; Ninomiya, F; Funabashi, M. Biobased contents of organic fillers and polycaprolactone composites with cellulose fillers measured by accelerator mass spectrometry based on ASTM D6866. J Polym Environ 2007, 15–4, 281–287. [Google Scholar]
- Funabashi, M; Ninomiya, F; Ohara, K; Kunioka, M. Biomass carbon ratio of biomass chemicals measured by accelerator mass spectrometry. Bull Chem Soc Jpn, (submitted for publication).
- Fujimaki, T. Precessability and properties of aliphatic polyesters, ‘BIONOLLE’, synthesized by polycondensation reaction. Polym. Degrad. Stab 1998, 59, 209–214. [Google Scholar]
- Mizukoshi, T. Bionolle—Approach to the more environmen-tally-friendly green plastics. The 2nd International Conference of Technology and Application of Biodegradable and Biobased Plastics (ICTABP2), Hangzhou, China; 2006; p. 123. [Google Scholar]
- Kato, S; Tsukuhara, T; Kishimoto, M; Nagaya, I. Development of green sustainable plastic (GS Pla). The 2nd International Conference of Technology and Application of Biodegradable and Biobased Plastics (ICTABP2), Hangzhou, China; 2006; p. 96. [Google Scholar]
- Shitani, N; Kato, S. Green sustainable plastic (GS Pla) and GS Pla-degrading enzyme from fungus. The First Asian-Oceanian Conference on Green & Sustainable Chemistry (GSC-AON 2007), Tokyo, Japan; 2007; p. 235. [Google Scholar]
- Kunioka, M; Ninomiya, F; Funabashi, M. Biodegradation of poly(lactic acid) powders proposed as the reference test materials for the international standard of biodegradation evaluation methods. Polym. Degrad. Stab 2006, 91, 1919–1928. [Google Scholar]
- Funabashi, M; Ninomiya, F; Kunioka, M. Biodegradation of polycaprolactone powders proposed as reference test materials for international standard of biodegradation evaluation method. J. Polym. Environ 2007, 15, 7–17. [Google Scholar]
- Maeda, H; Yamagata, Y; Abe, K; Hasegawa, F; Macheda, M; Ishioka, R; Gomi, K; Nakajima, T. Purification and characterization of a biodegradable plastic-degrading enzyme from Aspergillus oryzae. Appl. Microbiol. Biotechnol 2005, 67, 778–788. [Google Scholar]
- Masaki, K; Kamini, NR; Ikeda, H; Iefuji, H. Cutinase-like enzyme from the yeast Cryptococcus sp. strain S-2 hydrolyzes polylactic acid and other biodegradable plastics. Appl. Environ. Microbiol 2005, 71, 7548–7550. [Google Scholar]
- Tominaga, Y; Matsukawa, K; Kitagawa, K; Nakayama, A. Field test of soil biodegradability of biodegradable plastics in Japan. The 10th Pacific Basin Conference on Hazardous Waste at Okayama, Okayama, Japan; 2001; pp. 253–256. [Google Scholar]








| Analysis | YK-6 |
|---|---|
| Total dry solids (%)a) | 42 |
| Volatile solids (%)b) | 56 |
| pH of compost solution | 6.9 |
| Total organic carbon amount (%) | 9.1 |
| Total nitrogen amount (%) | 1.7 |
| C/N ratio | 5.0 |
| Test period (day) (center date (day)) | Evolved CO2a) (g) from sample vessel | Measured pMCb) (%) | Estimated pMCc) (%) | CO2 amount (g) | Biodegradabilityf) (%) | ||
|---|---|---|---|---|---|---|---|
| respirationd) | PBS biodegradatione) | in period | Total | ||||
| Blank(0–3) | 109.87 | ||||||
| 0–3 (1.5) | 1.39 | 91.87 | 101.95 | 1.29 | 0.10 | 0.5 | 0.5 |
| 3–6 (4.5) | 1.14 | 98.45 | 1.03 | 0.11 | 0.5 | 1.0 | |
| 6–9 (8.5) | 1.44 | 97.04 | 94.81 | 1.24 | 0.20 | 1.0 | 2.0 |
| 9–12 (10.5) | 1.12 | 92.02 | 0.94 | 0.18 | 0.9 | 2.9 | |
| 12–14 (13) | 0.99 | 88.19 | 0.79 | 0.20 | 1.0 | 3.8 | |
| 14–18 (16) | 1.67 | 83.44 | 83.20 | 1.26 | 0.41 | 2.0 | 5.8 |
| 18–21 (19.5) | 1.42 | 84.00 | 77.00 | 1.00 | 0.42 | 2.1 | 7.9 |
| 21–24 (22.5) | 1.76 | 69.05 | 71.54 | 1.15 | 0.61 | 3.0 | 10.9 |
| 24–28 (26) | 1.83 | 65.21 | 1.09 | 0.74 | 3.6 | 14.5 | |
| 28–32 (30) | 2.32 | 51.77 | 58.29 | 1.23 | 1.09 | 5.3 | 19.8 |
| 32–36 (34) | 2.32 | 52.05 | 1.10 | 1.22 | 6.0 | 25.8 | |
| 36–38 (37) | 1.67 | 48.12 | 48.03 | 0.73 | 0.94 | 4.6 | 30.4 |
| 38–60 (49) | 6.67 | 42.98 | 40.60 | 2.46 | 4.21 | 20.5 | 50.9 |
| 60–74 (67) | 3.08 | 70.69, 69.53 | 71.53 | 2.01 | 1.07 | 5.3 | 56.2 |
| Total | 28.82 | 17.32 | 11.50 | ||||
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kunioka, M.; Ninomiya, F.; Funabashi, M. Biodegradation of Poly(butylene succinate) Powder in a Controlled Compost at 58 °C Evaluated by Naturally-Occurring Carbon 14 Amounts in Evolved CO2 Based on the ISO 14855-2 Method. Int. J. Mol. Sci. 2009, 10, 4267-4283. https://doi.org/10.3390/ijms10104267
Kunioka M, Ninomiya F, Funabashi M. Biodegradation of Poly(butylene succinate) Powder in a Controlled Compost at 58 °C Evaluated by Naturally-Occurring Carbon 14 Amounts in Evolved CO2 Based on the ISO 14855-2 Method. International Journal of Molecular Sciences. 2009; 10(10):4267-4283. https://doi.org/10.3390/ijms10104267
Chicago/Turabian StyleKunioka, Masao, Fumi Ninomiya, and Masahiro Funabashi. 2009. "Biodegradation of Poly(butylene succinate) Powder in a Controlled Compost at 58 °C Evaluated by Naturally-Occurring Carbon 14 Amounts in Evolved CO2 Based on the ISO 14855-2 Method" International Journal of Molecular Sciences 10, no. 10: 4267-4283. https://doi.org/10.3390/ijms10104267