Dendronized Polyimides Bearing Long-Chain Alkyl Groups and Their Application for Vertically Aligned Nematic Liquid Crystal Displays
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Measurements
2.3. Synthesis of dendritic diamine monomers bearing long-chain alkyl groups
2.3.1. Dendritic building blocks
2.3.2. 12G1-AG-Dibromo precursor
2.3.3. 12G1-AG-Terphenyldiamine
2.3.4. 12G2-AG-Dibromo precursor
2.3.5. 12G2-AG-Terphenyldiamine
2.4. Preparation of poly(amic acid)s and polyimides
- Polyimide Based on BTDA /12G1-AG-Terphenyldiamine /DDE (100/50/50). IR (ATR): 2925, 2853 (C-H), 1718 (C=O), 1195, 1117 (-O-) cm−1.
- Polyimide Based on BTDA /12G1-AG-Terphenyldiamine /DDE (100/25/75). IR (ATR): 2924, 2854 (C-H), 1719 (C=O), 1203, 1116 (-O-) cm−1.
- Polyimide Based on BTDA /12G2-AG-Terphenyldiamine. 1H-=NMR (CDCl3, δ, ppm): 0.76–0.93 (m, CH3), 1.08–1.51 (m, CH3(CH2)9), 1.51–1.87 (m, CH2CH2OAr), 3.57–4.105 (m, CH2OAr), 6.40–6.80 (m, ArH), 7.07–8.48 (m, ArH); IR (ATR): 2922, 2853 (C-H), 1719 (C=O), 1190, 1110 (-O-) cm−1.
- Polyimide Based on BTDA /12G2-AG-Terphenyldiamine /DDE (100/75/25). 1H-NMR (CDCl3, δ, ppm): 0.74–0.93 (m, CH3), 1.11–1.57 (m, CH3(CH2)9), 1.57–1.88 (m, CH2CH2OAr), 3.51–4.08 (m, CH2OAr), 6.40–6.77 (m, ArH), 7.08–8.48 (m, ArH); IR (ATR): 2923, 2853 (C-H), 1719 (C=O), 1232, 1115 (-O-) cm−1.
- Polyimide Based on BTDA /12G2-AG-Terphenyldiamine /DDE (100/50/50). IR (ATR): 2924, 2853 (C-H), 1721 (C=O), 1193, 1115 (-O-) cm−1.
- Polyimide Based on 6FDA/ 12G1-AG-Terphenyldiamine. 1H-NMR (CDCl3, δ, ppm): 0.77–0.93 (m, CH3), 1.13–1.43 (m, CH3(CH2)8), 1.39–1.54 (m, CH2CH2CH2OAr), 1.57–1.88 (m, CH2CH2OAr), 3.88–4.14 (m, CH2OAr), 7.00–7.17 (m, ArH), 7.20–8.05 (m, ArH); IR (ATR): 2925, 2854 (C-H), 1725 (C=O), 1190, 1110 (-O-) cm−1.
- Polyimide Based on 6FDA/ 12G2-AG-Terphenyldiamine. 1H-NMR (CDCl3, δ, ppm): 0.79–0.98 (m, CH3), 1.16–1.35 (m, CH3(CH2)8), 1.35–1.54 (m, CH2CH2CH2OAr), 1.63–1.96 (m, CH2CH2OAr), 3.67–4.11 (m, CH2OAr), 6.54–6.57 (m, ArH), 7.05–8.17 (m, ArH); IR (ATR): 2922, 2853 (C-H), 1720 (C=O), 1191, 1110 (-O-) cm−1.
3. Results and Discussion
3.1. Monomer synthesis
3.2. Polymer synthesis
3.3. Polymer characterization and properties
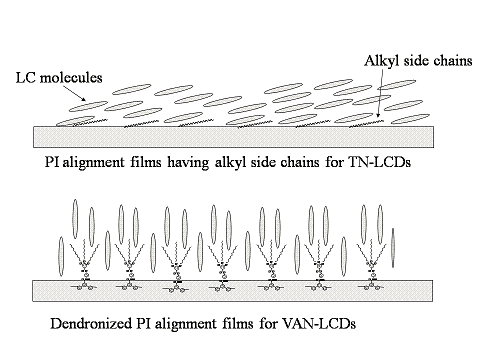
3.4. Alignment layer application for VAN-LCDs
4. Conclusions
Acknowledgments
References and Notes
- Mittal, KL (Ed.) Polyimides; Synthesis, Characterization and Applications; Plenum Press: New York, NY, USA, 1984; Volume 1&2.
- Ghosh, MK; Mittal, KL (Eds.) Polyimides; Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996.
- Mittal, KL (Ed.) Polyimides and Other High Temperature Polymers; VSP/Brill: Leiden, The Netherlands, 2005; Volume 3.
- Mittal, KL (Ed.) Polyimides and Other High Temperature Polymers; VSP/Brill: Leiden, The Netherlands, 2007; Volume 4.
- Mittal, KL. Polyimides and Other High Temperature Polymers; VSP/Brill: Leiden, The Netherlands, 2009; Volume 5. [Google Scholar]
- Hariharan, R; Sarojadevi, M. Synthesis and Characterization of Organo-soluble Fluorinated Polyimides. Polym. Int 2007, 56, 22–31. [Google Scholar]
- Zhao, XJ; Liu, JG; Rui, JM; Fan, L; Yang, SY. Synthesis and Characterization of Organosoluble Polyfluorinated Polyimides Derived from 3,3,5,5-Tetrafluoro-4,4-diaminodiphenylmethane and Various Aromatic Dianhydrides. J. Appl. Polym. Sci 2007, 103, 1442–1449. [Google Scholar]
- Li, W; Zhang, S; Chen, G; Zhang, Q; Li, W; Chen, G; Zhang, Q. A new class of high Tg and organosoluble polynaphthalimides. Polymer 2007, 48, 3082–3089. [Google Scholar]
- Qiu, Z; Zhang, S; Li, W; Qiu, Z; Li, W. Synthesis and Gas Permeability of Novel Fluorinated Poly(phenylene-co-naphthalimide)s. J. Appl. Polym. Sci 2007, 104, 2395–2402. [Google Scholar]
- Han, X; Xiao, C; Tian, Y; Wang, L. Formation of Honeycomb Films Based on a Soluble Polyimide Synthesized from 2,2′-Bis[4-(3,4-dicarboxyphenoxy)phenyl] hexafluoropropane dianhydride and 3,3′-Dimethyl-4,4′-diaminodiphenylmethane. J. Appl. Polym. Sci 2008, 107, 618–623. [Google Scholar]
- Choi, H; Kim, S-Y; Chung, I-S; Hong, K; Park, C-E. Soluble Polyimides from Unsymmetrical Diamine Containing Benzimidazole Ring and Trifluoromethyl Pendent Group. Polymer 2008, 49, 2644–2649. [Google Scholar]
- Li, Z; Liu, J; Gao, Z; Yin, Z; Fan, L; Yang, S. Organo-soluble and Transparent Polyimides Containing Phenylphosphine Oxide and Trifluoromethyl Moiety: Synthesis and Characterization. Eur. Polym. J 2009, 45, 1139–1148. [Google Scholar]
- Chung, CL; Lee, WF; Lin, CH; Hsiao, SH. Highly Soluble Fluorinated Polyimides Based on an Asymmetric Bis(ether amine): 1,7-Bis(4-amino-2-trifluoromethylphenoxy)naphthalene. J. Polym. Sci. A: Polym. Chem 2009, 47, 1756–1770. [Google Scholar]
- Qiu, Z; Chen, G; Zhang, Q; Zhang, S; Qiu, Z; Chen, G; Zhang, Q. Synthesis and Gas Transport Property of Polyimide from 2,2′-Disubstituted biphenyltetracarboxylic dianhydrides (BPDA). Eur. Polym. J 2007, 43, 194–204. [Google Scholar]
- Koohmareh, GA. New Organo-Soluble Polyimides Based on a New Dianhydride: 4-(4-t-Butyl-phenyl)-2,6-bis(3,4-phenyl dicarboxylic acid anhydride) pyridine. Des. Monomers Polym 2007, 10, 517–525. [Google Scholar]
- Mathews, AS; Kim, I; Ha, SC. Fully Aliphatic Polyimides from Adamantane-Based Diamines for Enhanced Thermal Stability, Solubility, Transparency, and Low Dielectric Constant. J. Appl. Polym. Sci 2006, 102, 3316–3326. [Google Scholar]
- Zhang, Q; Chen, G; Zhang, S; Zhang, Q; Chen, G. Snthesis and Properties of Novel Soluble Polyimides Having a Spirobisindane-linked Dianhydride Unit. Polymer 2007, 48, 2250–2256. [Google Scholar]
- Cao, S; Jin, Y; Xu, S; Yang, M; Xu, S; Bai, F. Highly Soluble Diphenylfluorene-based Cardo Copolyimides Containing Perylene Units. Polym. Adv. Technol 2006, 17, 556–561. [Google Scholar]
- Yang, C-P; Chen, Y-Y; Hsiao, S-H. Synthesis and Properties of Organosoluble Polynaphthalimides Bearing Ether Linkages and Phthalide Cardo Groups. J. Appl. Polym. Sci 2007, 104, 1104–1109. [Google Scholar]
- Wang, X; Li, Y; Zhang, S; Ma, T; Shao, Y; Zhao, X. Synthesis and Characterization of Novel Polyimides Derived from Pyridine-bridged Aromatic Dianhydride and Various Diamines. Eur. Polym. J 2006, 42, 1229–1239. [Google Scholar]
- Wang, X; Li, Y; Gong, C; Zhang, S; Ma, T. Synthesis and Characterization of Novel Soluble Pyridine-Containing Polyimides Based on 4-Phenyl-2,6-Bis[4-(4-Aminophenoxy)phenyl]-Pyridine and Various Aromatic Dianhydrides. J. Appl. Polym. Sci 2007, 104, 212–219. [Google Scholar]
- Anannarukan, W; Tantayanon, S; Zhang, D; Harris, FW; Aleman, EA; Modarelli, DA. Soluble Polyimides Containing Trans-diaminotetraphenylporphyrin: Synthesis and Photoinduced Electron Transfer. Polymer 2006, 47, 4936–4945. [Google Scholar]
- Xu, S; Cao, S; Xu, S; Yang, M. Synthesis and Optical Properties of Two Series of Soluble Acridine-containing Copolyimides. Polymer 2007, 48, 2241–2249. [Google Scholar]
- Chen, S; Tanaka, K; Kita, H; Okamoto, K; Yin, Y. Synthesis and Properties of Novel Side-chain-sulfonated Polyimides from Bis[4-(4-aminophenoxy)-2-(3-sulfobenzoyl)]phenyl Sulfone. Polymer 2006, 47, 2660–2669. [Google Scholar]
- Hu, Z; Yin, Y; Chen, S; Yamada, O; Tanaka, K; Kita, H; Okamoto, K. Synthesis and Properties of Novel Sulfonated (Co)polyimides Bearing Sulfonated Aromatic Pendant Groups for PEFC Applications. J. Polym. Sci. A: Polym. Chem 2006, 44, 2862–2872. [Google Scholar]
- Qiu, Z; Wu, S; Zhang, S; Qiu, Z; Wu, S; Li, Z; Li, Z; Xing, W; Liu, C. Sulfonated Poly(arylene-co-naphthalimide)s Synthesized by Copolymerization of Primarily Sulfonated Monomer and Fluorinated Naphthalimide Dichlorides as Novel Polymers for Proton Exchange Membranes. Macromolecules 2006, 39, 6425–6432. [Google Scholar]
- Li, Y; Jin, R; Wang, Z; Cui, Z; Xing, W; Gao, L; Li, Y; Cui, Z. Synthesis and Properties of Novel Sulfonated Polyimides Containing Binaphthyl Groups as Proton-exchange Membranes for Fuel Cells. J. Polym. Sci. A: Polym. Chem 2007, 45, 222–231. [Google Scholar]
- Sasthav, JR; Harris, FW. Internal Plasticization of Polyimides with Alkyl 3,5-Diaminobenzoate Compounds. Polymer 1995, 36, 4911–4917. [Google Scholar]
- Lee, KW; Paek, SH; Lien, A; Durning, C; Fukuro, H. Microscopic Molecular Reorientation of Alignment Layer Polymer Surfaces Induced by Rubbing and Its Effects on LC Pretilt Angles. Macromolecules 1996, 29, 8894–8899. [Google Scholar]
- Lee, C; Woo, TH; Lee, M. Effect of Alkyl Side Chain of Soluble Polyimide Orienting Layer on Liquid Crystal Alignment. Mol. Cryst. Liq. Cryst 1998, 316, 205–208. [Google Scholar]
- Ban, BS; Rin, YN; Kim, YB. Pretilt Angle for BTDA, PMDA, and CPDA Series Polyimides with Various Side Chain Length. Liquid Cryst 2000, 27, 125–130. [Google Scholar]
- Jung, JT; Yi, MH; Kwon, SK; Choi, KY. Aromatic Polyimides Containing Pendant Alkyl Groups for Liquid Crystal Alignment Layers. Mol. Cryst. Liq. Cryst 1999, 333, 1–13. [Google Scholar]
- Li, L; Yin, J; Sui, Y; Xu, HJ; Fang, JH; Zhu, ZK; Wang, ZG. Preparation and Properties of High-Pretilt-Angle Polyimides Based on an Alicyclic Dianhydride and Long Alkyloxy Side-group-containing Diamines. J. Polym. Sci., A: Polym. Chem 2000, 38, 1943–1950. [Google Scholar]
- Lee, SJ; Jung, JC; Lee, SW; Ree, M. Synthesis of Novel Polypyromellitimides with n-Alkyloxy Side Chains and Their Liquid-crystal Aligning Property. J Polym Sci, A: Polym Chem 2004, 42, 3130–3142. [Google Scholar]
- Tsuda, Y; Tanaka, Y; Kamata, K; Hiyoshi, N; Mataka, S; Matsuki, Y; Nishikawa, M; Kawamura, S; Bessho, N. Soluble Polyimides Based on 2,3,5-Tricarboxycyclopentyl Acetic Dianhydride. Polym. J 1997, 29, 574–579. [Google Scholar]
- Tsuda, Y; Etou, K; Hiyoshi, N; Nishikawa, M; Matsuki, Y; Bessho, N. Soluble Copolyimides Based on 2,3,5-Tricarboxycyclopentyl Acetic Dianhydride and Conventional Aromatic Tetracarboxylic Dianhydrides. Polym. J 1998, 30, 222–238. [Google Scholar]
- Tsuda, Y; Kuwahara, R; Fukuda, K; Ueno, K; Oh, J-M. Soluble Polyimides and Copolyimides Based on Alicyclic Dianhydride Having Cyclohexene and Tetralin Moieties. Polym. J 2005, 37, 126–132. [Google Scholar]
- Tsuda, Y; Kawauchi, T; Hiyoshi, N; Mataka, S. Soluble Polyimides Based on Alkyldiaminobenzophenone. Polym J 2000, 32, 594–601. [Google Scholar]
- Tsuda, Y; Kanegae, K; Yasukouchi, S. Soluble Polyimides Based on Alkyloxydiaminobenzene. Polym. J 2000, 32, 941–947. [Google Scholar]
- Tsuda, Y; Kojima, M; Oh, JM. Soluble Polyimides Based on Diaminobenzoic Acid Alkylester. Polymer J 2006, 38, 1043–1054. [Google Scholar]
- Tsuda, Y; Kojima, M; Matsuda, T; Oh, JM. Soluble Polyimides Based on Long-chain Alkyl Groups via Amide Linkages. Polym. J 2008, 40, 354–366. [Google Scholar]
- Tsuda, Y; Kuwahara, R; Oh, JM. Polyimides Having Dendron Side Chains. Trans. Mater. Res. Soc. Jpn 2004, 29, 267–270. [Google Scholar]
- Tsuda, Y; Yoshida, T; Kakoi, T. Synthesis of Soluble Polyimides Based on Alicyclic Dianhydride in Ionic Liquids. Polym. J 2005, 38, 88–90. [Google Scholar]
- Balagurusamy, VSK; Ungar, G; Percec, V; Johansson, G. Rational Design of the First Spherical Supramolecular Dendrimers Self-Organized in a Novel Thermotropic Cubic Liquid-Crystalline Phase and the Determination of Their Shape by X-ray Analysis. J Am Chem Soc 1997, 119, 1539–1555. [Google Scholar]
- Percec, V; Cho, W-D; Moeller, M; Prokhorova, SA; Ungar, G; Yeardley, DJP. Design and Structural Analysis of the First Spherical Monodendron Self-Organizable in a Cubic Lattice. J. Am. Chem. Soc 2000, 122, 4249–4250. [Google Scholar]
- Kaneda, T; Katsura, T; Nakagawa, K; Makino, H; Horio, M. High-Strength-High-Modulus Polyimide Fibers I. One-Step Synthesis of Spinnable Polyimides. J. Appl. Polym. Sci 1986, 32, 3133–3149. [Google Scholar]
- Inoue, H; Sasaki, Y; Ogawa, T. Comparison of One-Pot and Two-Step Polymerization of Polyimide from BPDA/ODA. J. Appl. Polym. Sci 1996, 60, 123–131. [Google Scholar]
- Takekoshi, T. Synthesis of polyimides. In Polyimides; Fundamentals and Applications; Ghosh, MK, Mittal, KL, Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 7–48. [Google Scholar]
- Wang, DH; Shen, Z; Guo, M; Cheng, SZD; Harris, FW. Synthesis and Properties of Polyimides Containing Multiple Alkyl Side Chains. Macromolecules 2007, 40, 889–900. [Google Scholar]
- Yang, CP; Su, YY; Hsu, MY. Organo-Soluble and Lightly-Colored Fluorinated Polyimides Based on 2,2-Bis[4-(3,4-dicarboxyphenoxy)phenyl]hexafluoropropane Dianhydride and Aromatic Bis(ether amine)s Bearing Pendent Trifluoromethyl Groups. Polym. J 2006, 38, 132–144. [Google Scholar]











| Monomer | Polymerization solvent | Poly(amic acid) | Polyimide | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dianhydridea, b | Diaminec | Comonomerd | ηinhi | Solubilityj | ηinhi | Molecular Weightk | |||
| (mol%) | (mol%) | dLg−1 | dLg−1 | Mn | Mw | Mw/Mn | |||
| BTDA | 12G1 | NMPe | 0.31 | Insoluble | |||||
| BTDA | 12G1 (50) | DDE (50) | NMP | 1.00 | Insoluble | ||||
| BTDA | 12G1 | m-cresolf | Insoluble | ||||||
| BTDA | 12G1 (75) | DDE (25) | m-cresol | Insoluble | |||||
| BTDA | 12G1 (50) | DDE (50) | m-cresol | Soluble | 0.56 | ||||
| BTDA | 12G1 (25) | DDE (75) | m-cresol | Soluble | 0.78 | ||||
| BTDA | DDE | m-cresol | Insoluble | ||||||
| BTDA | 12G2 | Pyridineg | Soluble | 0.06 | 11700 | 12800 | 1.3 | ||
| BTDA | 12G2(75) | DDE (25) | Pyridine | Soluble | 0.12 | 16500 | 20800 | 1.3 | |
| BTDA | 12G2 (50) | DDE (50) | Pyridine | Soluble | 0.22 | ||||
| BTDA | 12G2 (25) | DDE (75) | Pyridine | Insoluble | |||||
| 6FDA | 12G1 | NMP | 0.36 | Soluble | 0.28 | 22200 | 43600 | 1.9 | |
| 6FDA | 12G2 | NMP/THFh | Soluble | 0.12 | 12400 | 20100 | 1.6 | ||
| Polymer Compotition | Solubility | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dianhydrides | Diamine | Comonomer | Solubility in various solvents (5 wt%)a | ||||||||
| (mol %) | (mol %) | NMP | DMF | DMSO | CH2Cl2 | CHCl3 | THF | Toluene | Pyridine | m-Cresol | |
| BTDA | 12G1 (50) | DDE (50) | S(h) | I | I | I | I | I | I | I | S(h) |
| BTDA | 12G1 (25) | DDE (75) | S(h) | I | I | I | I | I | I | I | S(h) |
| BTDA | 12G2 | I | I | I | S(h) | S | S | S | S(h) | S(h) | |
| BTDA | 12G2(75) | DDE (25) | PS(h) | I | I | S | S | S | S | S(h) | S(h) |
| BTDA | 12G2 (50) | DDE (50) | PS(h) | I | I | I | I | I | I | S(h) | S(h) |
| 6FDA | 12G1 | S | S | I | PS | S | S | S | S | S | |
| 6FDA | 12G2 | I | I | I | S | S | S | S | S | S | |
| Polymer Compotition | Thermal Property | ||||
|---|---|---|---|---|---|
| Dianhydrides | Diamine | Comonomer | Tga | Td10b | |
| (mol%) | (mol%) | °C | °C in Air | °C in N2 | |
| BTDA | 12G1 (50) | DDE (50) | not observed | 455 | 449 |
| BTDA | 12G1 (25) | DDE (75) | not observed | 442 | 448 |
| BTDA | 12G2 | 249 | 365 | 381 | |
| BTDA | 12G2(75) | DDE (25) | not observed | 362 | 384 |
| BTDA | 12G2 (50) | DDE (50) | 269 | 349 | 386 |
| 6FDA | 12G1 | 311 | 436 | 440 | |
| 6FDA | 12G2 | 271 | 441 | 375 | |
| ITEM | PIA-DEN | TN mode | |
|---|---|---|---|
| Pretilt angle (°) | >89 | 4~6 | |
| Surface energy (dyn/cm2)a | 39 | 48 | |
| VHR (%) | 25°C | >99 | >99 |
| 60°C | >98 | >95 | |
| Response time (ms) | <25 | <30 | |
| Contrast ratio | 580 | 250 | |
| Residual DC (mv) | <200 | <200 | |
| Image sticking | <1 | <1 | |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsuda, Y.; OH, J.M.; Kuwahara, R. Dendronized Polyimides Bearing Long-Chain Alkyl Groups and Their Application for Vertically Aligned Nematic Liquid Crystal Displays. Int. J. Mol. Sci. 2009, 10, 5031-5053. https://doi.org/10.3390/ijms10115031
Tsuda Y, OH JM, Kuwahara R. Dendronized Polyimides Bearing Long-Chain Alkyl Groups and Their Application for Vertically Aligned Nematic Liquid Crystal Displays. International Journal of Molecular Sciences. 2009; 10(11):5031-5053. https://doi.org/10.3390/ijms10115031
Chicago/Turabian StyleTsuda, Yusuke, Jae Min OH, and Renpei Kuwahara. 2009. "Dendronized Polyimides Bearing Long-Chain Alkyl Groups and Their Application for Vertically Aligned Nematic Liquid Crystal Displays" International Journal of Molecular Sciences 10, no. 11: 5031-5053. https://doi.org/10.3390/ijms10115031