Immune Response among Patients Exposed to Molds
Abstract
:1. Introduction
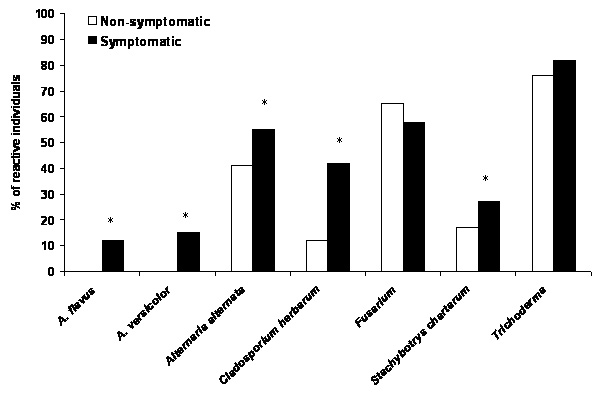
2. Results and Discussion
3. Experimental Section
4. Conclusions
References and Notes
- Khalili, B; Montanaro, MT; Bardana, EJ, Jr. Inhalational mold toxicity: fact or fiction? A clinical review of 50 cases. Ann. Allergy Asthma Immunol 2005, 95, 239–246. [Google Scholar]
- Edmondson, DA; Nordness, ME; Zacharisen, MC; Kurup, VP; Fink, JN. Allergy and “toxic mold syndrome”. Ann. Allergy Asthma Immunol 2005, 94, 234–239. [Google Scholar]
- Portnoy, JM; Kennedy, K; Barnes, CS. Controversies regarding dampness and mold growth in homes. Allergy Asthma Proc 2007, 28, 257–258. [Google Scholar]
- Hope, AP; Simon, RA. Excess dampness and mold growth in homes: an evidence-based review of the aeroirritant effect and its potential causes. Allergy Asthma Proc 2007, 28, 262–270. [Google Scholar]
- Johanning, E. Indoor moisture and mold-related health problems. Eur. Ann. Allergy Clin. Immunol 2004, 36, 182–185. [Google Scholar]
- Fung, F; Hughson, WG. The fundamentals of mold-related illness: When to suspect the environment is making a patient sick. Postgrad. Med 2008, 120, 80–84. [Google Scholar]
- Hardin, BD; Kelman, BJ; Saxon, A. Adverse Human Health Effects Associated with Molds in the Indoor Environment. Evidence-based Statements.
- Bush, RK; Portnoy, JM; Saxon, A; Terr, AI; Wood, RA. The medical effects of mold exposure. J. Allergy Clin. Immunol 2006, 117, 326–333. [Google Scholar]
- Mazur, LJ; Kim, J. Committee on Environmental Health, American Academy of Pediatrics: Spectrum of noninfectious health effects from molds. Pediatrics 2006, 118, 1909–1926. [Google Scholar]
- Vojdani, A; Campbell, AW; Kashanian, A; Vojdani, E. Antibodies against molds and mycotoxins following exposure to toxigenic fungi in a water-damaged building. Arch. Environ. Health 2003, 58, 324–336. [Google Scholar]
- Hodgson, MJ; Morey, P; Leung, WY; Morrow, L; Miller, D; Jarvis, BB; Robbins, H; Halsey, JF; Storey, E. Building-associated pulmonary disease from exposure to Stachybotrys chartarum and Aspergillus versicolor. J. Occup. Environ. Med 1998, 40, 241–249. [Google Scholar]
- Barrios, C; Reijula, K; Kurup, VP. Indoor environment and mold-related illness. In Mold Allergy, Biology and Pathogenesis; Kurup, VP, Ed.; Research Signpost: Kerala, India, 2005; pp. 369–388. [Google Scholar]
- Barrios, CS; Fink, JN; Kurup, VP. Mycotoxins and mold induced illness (MII). In Recent Trends in Aerobiology, Allergy, & Immunology; Vijay, HM, Kurup, VP, Eds.; Research Signpost: Kerala, India, 2008; pp. 427–447. [Google Scholar]
- Gray, MR; Thrasher, JD; Crago, R; Madison, RA; Arnold, L; Campbell, AW; Vojdani, A. Mixed mold mycotoxicosis: immunological changes in humans following exposure in water-damaged buildings. Arch. Environ. Health 2003, 58, 410–420. [Google Scholar]
- Krouse, JH; Stachler, RJ; Shah, A. Current in vivo and in vitro screens for inhalant allergy. Otolaryngol. Clin. North Am 2003, 36, 855–868. [Google Scholar]
- Rea, WJ; Didriksen, N; Simon, TR; Pan, Y; Fenyves, EJ; Griffiths, B. Effects of toxic exposure to molds and mycotoxins in building-related illnesses. Arch. Environ. Health 2003, 58, 399–405. [Google Scholar]
- Malkin, R; Martinez, KI; Marinkowich, V; Wilcox, T; Wall, D; Biagini, R. The relationship between symptoms and IgG and IgE antibodies in an office environment. Environ. Res 1998, 76, 85–93. [Google Scholar]
- Immonen, J; Laitinen, S; Taskinen, T; Pekkanen, J; Nevalainen, A; Korppi, M. Mould-specific immunoglobulin G antibodies in students from moisture- and mould-damaged schools: A 3-year follow-up study. Pediatr. Allergy Immunol 2002, 13, 125–128. [Google Scholar]
- Portnoy, JM; Kwak, K; Dowling, P; VanOsdol, T; Barnes, C. Health effects of indoor fungi. Ann. Allergy Asthma Immunol 2005, 94, 313–319. [Google Scholar]
- Jaakkola, MS; Nordman, H; Piipari, R; Uitti, J; Laitinen, J; Karjalainen, A; Hahtola, P; Jaakkola, JJ. Indoor dampness and molds and development of adult-onset asthma: A population-based incident case-control study. Environ. Health Perspect 2002, 110, 543–547. [Google Scholar]
- Gent, JF; Ren, P; Belanger, K; Triche, E; Bracken, MB; Holford, TR; Leaderer, BP. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ. Health Perspect 2002, 110, 781–786. [Google Scholar]
- Jaakkola, JJ; Hwang, BF; Jaakkola, N. Home dampness and molds, parental atopy, and asthma in childhood: A six-year population-based cohort study. Environ. Health Perspect 2005, 113, 357–361. [Google Scholar]
- Taskinen, TM; Laitinen, S; Nevalainen, A; Vepsalainen, A; Meklin, T; Reiman, N; Korpi, M; Husman, T. Immunoglobulin G antibodies to moulds in school-children from moisture problem schools. Allergy 2002, 57, 9–16. [Google Scholar]
- Raunio, P; Pasanen, A-L; Husman, T; Virtanen, T. Bioaerosols, Fungi and Mycotoxins: Health Effects, Assessment, Prevention and Control; Johanning, E, Ed.; Eastern New York Occupational and Environmental Health Center: New York, NY, USA, 1999; p. 174. [Google Scholar]
- Yike, I; Distler, AM; Ziady, AG; Dearborn, DG. Mycotoxin adducts on human serum albumin: Biomarkers of exposure to Stachybotrys chartarum. Environ. Health Perspect 2006, 114, 1221–1226. [Google Scholar]
- Brasel, TL; Campbell, AW; Demers, RE; Ferguson, BS; Fink, J; Vojdani, A; Wilson, SC; Straus, DC. Detection of trichothecene mycotoxins in sera from individuals exposed to Stachybotrys chartarum in indoor environments. Arch. Environ. Health 2004, 59, 317–323. [Google Scholar]
- Hooper, DG; Bolton, VE; Guilford, FT; Straus, DC. Mycotoxin detection in human samples from patients exposed to environmental molds. Int. J. Mol. Sci 2009, 10, 1465–1475. [Google Scholar]
- Chapman, JA. Update on airborne mold and mold allergy. Allergy Asthma Proc 1999, 20, 289–292. [Google Scholar]
- Malling, HJ. Diagnosis of mold allergy. Clin. Rev. Allergy 1992, 10, 213–236. [Google Scholar]
- Calhoun, KH. Patterns of mold sensitivity in the subtropical Gulf Coast. Otolaryngol. Head Neck Surg 2004, 130, 306–311. [Google Scholar]
- Kurup, VP; Knutsen, AP; Moss, RB; Bansal, NK. Specific antibodies to recombinant allergens of Aspergillus fumigatus in cystic fibrosis patients with ABPA. Clin. Mol. Allergy 2006, 21, 4–11. [Google Scholar]
- Gugnani, HC; Reijula, KE; Kurup, VP; Fink, JN. Detection of IgG and IgE antibodies to Aspergillus fumigatus in human sera by immunogold assay. Mycopathologia 1990, 109, 33–40. [Google Scholar]
- Barrios, CS; Johnson, BD; Henderson, JD, Jr; Fink, JN; Kelly, KJ; Kurup, VP. The costimulatory molecules CD80, CD86, and OX40L are upregulated in Aspergillus fumigatus sensitized mice. Clin. Exp. Immunol 2005, 142, 242–250. [Google Scholar]
- Johnson, BD; Kurup, VP; Sussman, GL; Arif, SAM; Kelly, KJ; Beezhold, DH; Fink, JN. Purified and recombinant latex proteins stimulate peripheral blood lymphocytes of latex allergic patients. Int. Arch. Allergy Immunol 1999, 120, 270–279. [Google Scholar]
- Brasel, TL; Douglas, DR; Wilson, SC; Straus, DC. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins on particulates smaller than conidia. Appl. Environ. Microbiol 2005, 71, 114–122. [Google Scholar]
- Brasel, TL; Martin, JM; Carriker, CG; Wilson, SC; Straus, DC. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins in the indoor environment. Appl. Environ. Microbiol 2005, 71, 7376–7388. [Google Scholar]




| Characteristics | Children (n = 7) | Adults (n = 26) | Total (n = 33) |
|---|---|---|---|
| Gender | |||
| Male | 4 (57%) | 12 (46%) | 16 (48%) |
| Female | 3 (43%) | 14 (54%) | 17 (52%) |
| Age, mean (range), y | 13 (10–17) | 41 (18–57) | 35 (10–57) |
| Past medical history | |||
| Asthma | 0 (0%) | 7 (27%) | 7 (21%) |
| Allergic rhinitis* | 1 (14%) | 5 (19%) | 6 (18%) |
| Mold exposure site** | |||
| Home | 7 (100%) | 22 (85%) | 29 (88%) |
| School | 0 (0%) | 1 (4%) | 1 (3%) |
| Work/Office | 0 (0%) | 3 (11%) | 3 (9%) |
| Social history | |||
| Smoke exposure*** | 3 (43%) | 12 (46%) | 15 (45%) |
| Pets exposure**** | 17 (65%) | 6 (86%) | 23 (70%) |
| Symptoms | Children (n = 7) | Adults (n = 26) | Total (n = 33) |
|---|---|---|---|
| Rhinitis | 7 (100%) | 20 (77%) | 27 (82%) |
| Cough | 5 (71%) | 14 (54%) | 19 (58%) |
| Headache | 3 (43%) | 11 (42%) | 14 (42%) |
| Respiratory symptoms* | 4 (57%) | 20 (77%) | 24 (73%) |
| Ocular pruritus | 5 (71%) | 12 (46%) | 17 (52%) |
| CNS symptoms** | 0 (0%) | 10 (38%) | 10 (30%) |
| Fatigue | 0 (0%) | 12 (46%) | 12 (36%) |
| Gastrointestinal symptoms*** | 3 (43%) | 13 (50%) | 16 (48%) |
| Epistaxis | 0 (0%) | 1 (4%) | 1 (3%) |
| Dysuria | 0 (0%) | 4 (15%) | 4 (12%) |
| Mold Antigens for IDT** | Mold Antigens for SPT** | ||
|---|---|---|---|
| Mold Group I | 13 (39%) | Alternaria | 8(24%) |
| Aspergillus fumigatus | 4(12%) | ||
| Aspergillus niger | 0 (0%) | ||
| Botrytis cinerea | 0 (0%) | ||
| Candida albicans | 0 (0%) | ||
| Candida tropicalis | 0 (0%) | ||
| Cephalosporium | 0 (0%) | ||
| Cephalothecium roseum | 0 (0%) | ||
| Mold Group II | 16 (48%) | Chaetomium globosum | 0 (0%) |
| Cladosporium fulvum | 0 (0%) | ||
| Curvularia spicifera | 2 (6%) | ||
| Epicoccum nigrum | 2 (6%) | ||
| Fusarium oxysporum | 2 (6%) | ||
| Geotrichum candidum | 0 (0%) | ||
| Cliocladium fimbriatum | 0 (0%) | ||
| Helminthosporium | 1 (3%) | ||
| Mold Group III | 16 (48%) | Hormodendrum | 4 (12%) |
| Microsporum audouinii | 0 (0%) | ||
| Microporum canis | 0 (0%) | ||
| Mucor racemosus | 0 (0%) | ||
| Neurospora intermedia | 1 (3%) | ||
| Nigrospora oryzae | 2 (6%) | ||
| Paecilomyces variotii | 1 (3%) | ||
| Papularia montagnei | 0 (0%) | ||
| Mold Group IV | 11 (33%) | Penicillium notatum | 2 (6%) |
| Phoma destructive | 1 (3%) | ||
| Pullularia pullulans | 1 (3%) | ||
| Rhizopus stolonifer | 1 (3%) | ||
| Rhodotorula rubra | 1 (3%) | ||
| Saccharomyces | 0 (0%) | ||
| Scopulariopsis | 0 (0%) | ||
| Spondylocladium | 1 (3%) | ||
| Mold Group V | 6 (18%) | Sporotrichum | 1 (3%) |
| Stachybotrys chartarum | 1 (3%) | ||
| Stemphylium herbarum | 2 (6%) | ||
| Phycomyces | 0 (0%) | ||
| Syncephalastrum | 0 (0%) | ||
| Tetracoccosporium | 0 (0%) | ||
| Trichoderma rufa | 1 (3%) | ||
| Trichophyton rubrum | 1 (3%) | ||
| Total | 19 (58%) | 13 (39%) | |
| Controls | Negative Control | 0 (0%) | |
| Histamine | 33 (100%) | ||
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Edmondson, D.A.; Barrios, C.S.; Brasel, T.L.; Straus, D.C.; Kurup, V.P.; Fink, J.N. Immune Response among Patients Exposed to Molds. Int. J. Mol. Sci. 2009, 10, 5471-5484. https://doi.org/10.3390/ijms10125471
Edmondson DA, Barrios CS, Brasel TL, Straus DC, Kurup VP, Fink JN. Immune Response among Patients Exposed to Molds. International Journal of Molecular Sciences. 2009; 10(12):5471-5484. https://doi.org/10.3390/ijms10125471
Chicago/Turabian StyleEdmondson, David A., Christy S. Barrios, Trevor L. Brasel, David C. Straus, Viswanath P. Kurup, and Jordan N. Fink. 2009. "Immune Response among Patients Exposed to Molds" International Journal of Molecular Sciences 10, no. 12: 5471-5484. https://doi.org/10.3390/ijms10125471