Philosophical Basis and Some Historical Aspects of Systems Biology: From Hegel to Noble - Applications for Bioenergetic Research
Abstract
:Contents
- Systems Biology – new paradigm and new perspectives of biological research.
- Systems Biology and Hegel’s dialectic, some important steps in history.
- Application of the Systems Biology approach to metabolic studies. Metabolic compartmentation as system level property.
- Molecular System Bioenergetics: structural and dynamic organization of cellular energy metabolism: mitochondrial-cytoskeletal interactions, mitochondrial dynamics, energetic modules and regulation mechanisms.
- Mathematical models of energy metabolism, useful and not very useful.
Wherever there is movement, wherever there is life, Wherever anything is carried into effect in the actual word, there Dialectic is at work. It is also the soul of all knowledge which is truly scientific.Hegel’s Logic, Translated by William Wallace, Oxford University Press, Oxford UK, 2005, p. 116
1. Systems Biology – New Paradigm and New Perspectives of Biological Research
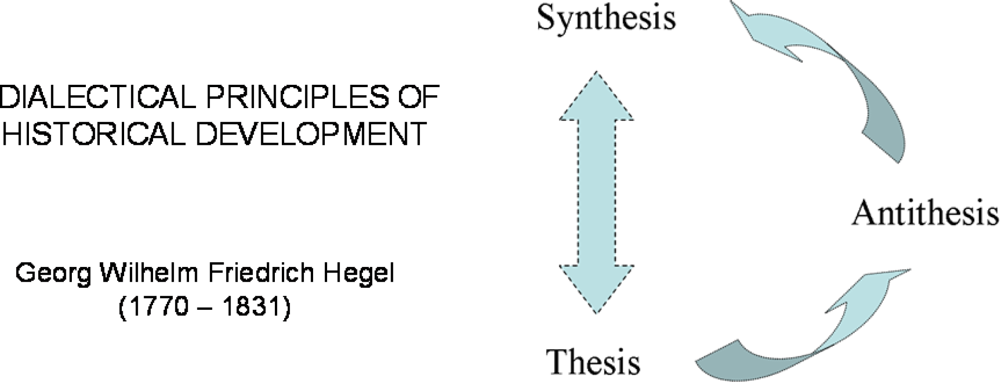
2. Hegel’s Dialectic and Systems Biology. Some Important Steps in History
2.1. Hegel’s dialectic laws
2.2. Claude Bernard and the theory of permanence of internal milieu – homeostasis
2.3. Cybernetics of Norbert Wiener and Systems Biology
2.4. Systems Biology: from Hegel to Noble
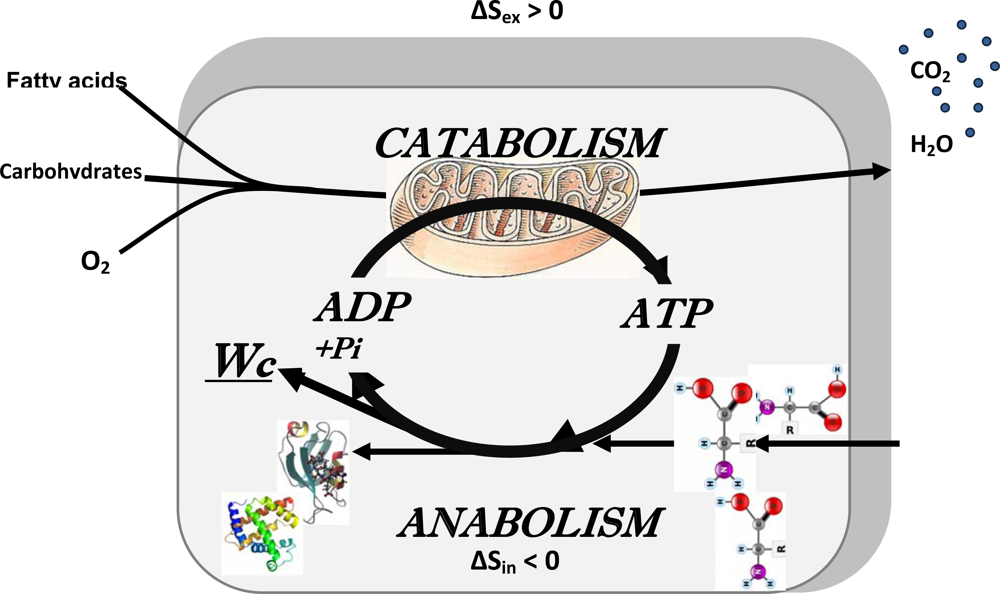
2.5. Erwin Schrödinger: negentropy production as a basis of metabolism, central role of bioenergetics
3. Application of the Systems Biology Approach to Metabolic Studies. Metabolic Compartmentation as System Level Property
4. Molecular System Bioenergetics: Structural and Dynamic Organization of Cellular Energy Metabolism, Mitochondrial-Cytoskeletal Interactions, Mitochondrial Dynamics, Energetic Modules and Regulatory Mechanisms
4.1. Unitary organization of energy metabolism and compartmentalized energy transfer in cardiac cells
4.2. Cardiac cells as highly organized metabolic systems
4.3. Unitary organization of energy metabolism versus mitochondrial reticulum
4.4. Excitation-contraction coupling and cardiac energetics: membrane energy sensing
4.5. Molecular system analysis of integrated mechanisms of regulation of fatty acid and glucose oxidation
5. Mathematical Models of Energy Metabolism, Useful and Not Very Useful
6. Conclusions
Acknowledgments
References and Notes
- Kitano, HE. Foundations of Systems Biology; The MIT Press: Cambridge, Massachusetts, USA, London, UK, 2001. [Google Scholar]
- Noble, D. The Music of Life Biology Beyond the Genome; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Boogerd, FC; Bruggeman, FJ; Hofmeyr, J-HS; Westerhoff, HV. Systems Biology Philosophical Foundations; Elsevier: Amsterdam, The Netherlands, Oxford, UK, 2007. [Google Scholar]
- Alberghina, L; Westerhoff, HV. Systems Biology Definitions and Perspectives; Springer – Verlag: Heidelberg, 2005. [Google Scholar]
- Klipp, E; Herwig, R; Kowald, A; Wierling, C; Lehrach, H. Systems Biology in Practice Concepts, Implementation and Applications; Wiley–VCH Verlag GmbH&Co KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Alon, U. An Introduction to Systems Biology Design Principles of Biological Circuits; Chapman&Hall/CRC: Boca Raton, USA, 2007. [Google Scholar]
- Saks, V. Molecular System Bioenergetics; Saks, V, Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 1–604. [Google Scholar]
- Palsson, BO. Systems Biology: Properties of Reconstructed Networks; Cambridge University Press: New York, USA, 2006. [Google Scholar]
- Szallasi, Z; Jörg Stelling, J; Periwal, V. System Modeling in Cellular Biology: From Concepts to Nuts and Bolts; Szallasi, Z, Jörg Stelling, J, Periwal, V, Eds.; MIT Press: Cambridge, USA, 2006. [Google Scholar]
- Kitano, H. Systems Biology: A Brief Overview. Science 2002, 295, 1662–1664. [Google Scholar]
- Noble, D. Modeling the Heart-from Genes to Cells to the Whole Organ. Science 2002, 295, 1678–1682. [Google Scholar]
- Bassingthwaighte, JB; Hunter, PJ; Noble, D. The Cardiac Physiome - Perspectives for the Future. Exp Physiol 2008. [Google Scholar]
- Noble, D. Claude Bernard, the First Systems Biologist, and the Future of Physiology. Exp Physiol 2008, 93(1), 16–26. [Google Scholar]
- Noble, D. Prologue: Mind over Molecule: Activating Biological Demons. Ann. N. Y. Acad. Sci 2008, 1123, xi–xix. [Google Scholar]
- Westerhoff, HV; Kolodkin, A; Conradie, R; Wilkinson, SJ; Bruggeman, FJ; Krab, K; van Schuppen, JH; Hardin, H; Bakker, BM; Moné, MJ; Rybakova, KN; Eijken, M; van Leeuwen, HJ; Snoep, JL. Systems Biology Towards Life in Silico: Mathematics of the Control of Living Cells. J. Math. Biol 2009, 58, 7–34. [Google Scholar]
- Bruggeman, FJ; Rossell, S; Van Eunen, K; Bouwman, J; Westerhoff, HV; Bakker, B. Systems Biology and the Reconstruction of the Cell: From Molecular Components to Integral Function. Subcell Biochem 2007, 43, 239–262. [Google Scholar]
- Hunter, P; Nielsen, P. Strategy for Integrative Computational Physiology. Physiology 2005, 20, 316–325. [Google Scholar]
- Ravasz, E; Somera, AL; Mongru, DA; Oltvai, ZN; Barabasi, AL. Hierarchical Organization of Modularity in Metabolic Networks. Science 2002, 297, 1551–1555. [Google Scholar]
- Barabasi, AL; Oltvai, ZN. Network Biology: Understanding the Cell’s Functional Organization. Nature Rev. Genet 2004, 24, 101–113. [Google Scholar]
- Bing, RJ. Cardiac Metabolism. Physiol. Rev 1965, 45, 171–213. [Google Scholar]
- Vignais, P. Science Expérimentale Et Connaissance Du Vivant. La Méthode Et Les Concepts; EDP Sciences: Les Ulis, France, 2006. [Google Scholar]
- Vignais, P. La Biologie Des Origines a Nos Jours; EDP Sciences: Les Ulis, France, 2001. [Google Scholar]
- Bernard, C. Introduction À L’étude De La Médicine Expérimentale; Flammarion: Paris, 1984. [Google Scholar]
- Wiener, N. Cybernetics or Control and Communication in the Animal and the Machine; The MIT Press: Cambridge, Massachussets, 1948. [Google Scholar]
- Schrödinger, E. What Is Life? Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Kuhn, TS. The Structure of Scientific Revolutions. University of Chicago: Chicago, USA, 1962. [Google Scholar]
- Hegel, GWF. The Encyclopedia Logic. Part I of the Encycopedia of Philosophical Sciences with the Zusätze; Hackett Publishing Company: Indianapolis/Cambridge, USA, 1991. [Google Scholar]
- Hegel’s Logic; The Clarendon Press: Oxford, UK, 1975.
- Hegel’s Philosophy of Nature Part II of the Encyclopedia of the Philosophical Sciences; Oxford University Press: Oxford, UK, 1970.
- Bertrand, R. History of Western Philosophy and Its Connection with Political and Social Circumstances from the Earliest Times to the Present Day; Routledge: London, UK, 1996; pp. 701–715. [Google Scholar]
- Prigogine, I; Strengers, I. La Nouvelle Alliance; Les Editions Gallimard: Paris, 1986. [Google Scholar]
- Nicolis, G; Prigogine, I. Self-Organization in Non-Equilibrium Systems; Wiley-Interscience: London, 1977. [Google Scholar]
- Aon, MA; Cortassa, S. Dynamic Biological Organization Fundamentals as Applied to Cellular Systems; Chapman and Hall: London, 1997. [Google Scholar]
- Schneider, ED; Sagan, D. Into the Cool Energy Flow, Thermodynamics and Life; The University of Chicago Press: Chicago, 2005. [Google Scholar]
- Saks, V; Beraud, N; Wallimann, T. Metabolic Compartmentation – a System Level Property of Muscle Cells: Real Problems of Diffusion in Living Cells. Int. J. Mol. Sci 2008, 9, 751–767. [Google Scholar]
- Agutter, PS; Malone, PC; Wheatley, DN. Intracellular Transport Mechanisms: A Critique of Diffusion Theory. J. Theor. Biol 1995, 176, 261–272. [Google Scholar]
- Agutter, PS; Malone, PC; Wheatley, DN. Diffusion Theory in Biology: A Relic of Mechanistic Materialism. J. Hist Biol 2000, 33, 71–111. [Google Scholar]
- Wheatley, DN. Diffusion Theory, the Cell and the Synapse. Biosystems 1998, 45, 151–163. [Google Scholar]
- Saks, V; Monge, C; Anmann, T; Dzeja, P. Integrated and Organized Cellular Energetic Systems: Theories of Cell Energetics, Compartmentation and Metabolic Channeling. In Molecular System Bioenergetics Energy for Life; Saks, V, Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 59–110. [Google Scholar]
- Welch, GR. On the Role of Organized Multienzyme Systems in Cellular Metabolism: A General Synthesis. Prog. Biophys. Mol. Biol 1977, 32, 103–191. [Google Scholar]
- Welch, GR. Organized Multienzyme Systems; Academic Press: Orlando, USA, 1985; pp. 1–447. [Google Scholar]
- Ovàdi, J. Cell Architecture and Metabolic Channeling; R.G. Landes Co: Austin, TX, USA, 1995. [Google Scholar]
- Ovàdi, J; Saks, V. On the Origin of Intracellular Compartmentation and Organized Metabolic Systems. Mol Cell Biochem 2004, 256/257, 5–12. [Google Scholar]
- Srivastava, DK; Bernhard, SA. Metabolite Transfer Via Enzyme-Enzymz Complexes. Science 1986, 234, 1081–1086. [Google Scholar]
- Qian, H; Elson, EL. Single-Molecule Enzymology: Stochastic Michaelis-Menten Kinetics. Biophys Chem 2002, 101/102, 565–576. [Google Scholar]
- Huang, X; Holden, HM; Raushel, FM. Channeling of Substrates and Intermediates in Enzyme-Catalyzed Reactions. Annu. Rev. Biochem 2001, 70, 149–180. [Google Scholar]
- Ovàdi, J; Srere, AP. Macromolecular Compartmentation and Channeling. Intern. Rev. Cytol 2000, 192, 255–280. [Google Scholar]
- Weiss, JN; Yang, L; Qu, Z. Network Perspectives of Cardiovascular Metabolism. J. Lipid Res 2006, 47, 2355–2366. [Google Scholar]
- Saks, V; Dzeja, P; Schlattner, U; Vendelin, M; Terzic, A; Wallimann, T. Cardiac System Bioenergetics: Metabolic Basis of Frank-Starling Law. J. Physiol 2006, 571, 253–273. [Google Scholar]
- Rosing, J; Slater, ES. The Value of G0 for the Hydrolysis of ATP. Biochim. Biophys. Acta 1972, 267, 275–290. [Google Scholar]
- Veech, RL; Lawson, JWR; Cornell, NW; Krebs, HA. Cytosolic Phosphorylation Potential. J. Biol. Chem 1979, 254, 6538–6547. [Google Scholar]
- Kammermeier, H; Schmidt, P; Jungling, E. Free Energy Change of ATP Hydrolysis: A Causal Factor of Early Hypoxic Failure of the Myocardium? J. Mol. Cell. Cardiol 1982, 14, 267–277. [Google Scholar]
- Yoshizaki, K; Seo, Y; Nishikawa, H; Morimoto, T. Application of Pulsed-Gradient 31p NMR on Frog Muscle to Measure the Diffusion Rates of Phosphorus Compounds in Cells. Biophys. J 1982, 38, 209–211. [Google Scholar]
- Kinsey, ST; Locke, BR; Benke, B; Moerland, TS. Diffusional Anisotropy Is Induced by Subcellular Barriers in Skeletal Muscle. NMR Biomed 1999, 12, 1–7. [Google Scholar]
- Vendelin, M; Eimre, M; Seppet, E; Peet, N; Andrienko, T; Lemba, M; Engelbrecht, J; Seppet, EK; Saks, VA. Intracellular Diffusion of Adenosine Phosphates Is Locally Restricted in Cardiac Muscle. Mol Cell Biochem 2004, 256/257, 229–241. [Google Scholar]
- Abraham, MR; Selivanov, VA; Hodgson, DM; Pucar, D; Zingman, LV; Wieringa, B; Dzeja, P; Alekseev, AE; Terzic, A. Coupling of Cell Energetics with Membrane Metabolic Sensing. Integrative Signaling through Creatine Kinase Phosphotransfer Disrupted by M-CK Gene Knock-Out. J. Biol. Chem 2002, 277, 24427–24434. [Google Scholar]
- Selivanov, VA; Alekseev, AE; Hodgson, DM; Dzeja, PP; Terzic, A. Nucleotide-Gated K-ATP Channels Integrated with Creatine and Adenylate Kinases: Amplification, Tuning and Sensing of Energetics Signals in the Compartmentalized Cellular Environment. Mol Cell Biol 2004, 256/257, 243–256. [Google Scholar]
- Kennedy, HJ; Pouli, AE; Ainscow, EK; Jouaville, LS; Rizzuto, R; Rutter, GA. Glucose Generates Sub-Plasma Membrane ATP Microdomains in Single Islet -Cells. J. Biol. Chem 1999, 274, 13291–13291. [Google Scholar]
- Neubauer, S. The Failing Heart-an Engine out of Fuel. N. Engl. J. Med 2007, 356, 1140–1151. [Google Scholar]
- Fawcett, DW; McNutt, NS. The Ultrastructure of the Cat Myocardium. I. Ventricular Papillary Muscle. J. Cell Biol 1969, 42, 1–45. [Google Scholar]
- Aon, M; Cortassa, S; O’Rourke, B. Percolation and Criticality in a Mitochondrial Network. Proc. Natl. Acad. Sci. USA 2004, 101, 4447–4452. [Google Scholar]
- Vendelin, M; Beraud, N; Guerrero, K; Andrienko, T; Kuznetsov, AV; Olivares, J; Kay, L; Saks, VA. Mitochondrial Regular Arrangement in Muscle Cells: A “Crystal-Like” Pattern. Am. J. Physiol. Cell Physio 2005, 288, C757–C767. [Google Scholar]
- Bereiter-Hahn, J; Voth, M. Dynamics of Mitochondria in Living Cells: Shape Changes, Dislocations, Fusion and Fission of Mitochondria. Microsci. Res. Tech 1994, 27, 198–219. [Google Scholar]
- Yi, M; Weaver, D; Hajnocsky, G. Control of Mitochondrial Motility and Distribution by the Calcium Signal: A Homeostatic Circuit. J. Cell Biol 2004, 167, 661–672. [Google Scholar]
- Rube, DA; van den Bliek, AM. Mitochondrial Morphology Is Dynamic and Varied. Mol. Cell. Biochem 2004, 256/257, 331–339. [Google Scholar]
- Mitchell, P. Coupling of Phosphorylation to Electron Transfer by a Chemi-Osmotic Type of Mechanism. Nature 1961, 191, 144–148. [Google Scholar]
- Gudbjarnason, S; Mathes, P; Raven, KG. Functional Compartmentation of ATP and Creatine Phosphate in Heart Muscle. J. Mol. Cell. Cardiol 1970, 1, 325–339. [Google Scholar]
- Kupriyanov, VV; Lakomkin, VL; Korchazhkina, OV; Steinschneider, AYa; Kapelko, VI; Saks, VA. Control of Cardiac Energy Turnover by Cytoplasmic Phosphates: 31p-NMR Study. Am. J. Physiol 1991, 261, 45–53. [Google Scholar]
- McLellan, G; Weisberg, A; Winegrad, S. Energy Transport from Mitochondria to Myofibril by a Creatine Phosphate Shuttle in Cardiac Cells. Am. J. Physiol 1983, 254, C423–C427. [Google Scholar]
- Saks, VA; Rosenshtraukh, LV; Undrovinas, A; Smirnov, VN; Chazov, E. Studies of Energy Transport in Heart Cells. Intracellular Creatine Content as a Regulatory Factor of Frog Heart Energetics and Force of Contraction. Biochem. Med 1976, 16, 21–36. [Google Scholar]
- Saks, VA; Khuchua, ZA; Vasilyeva, EV; Belikova; Yu, O; Kuznetsov, A. Metabolic Compartmentation and Substrate Channeling in Muscle Cells. Role of Coupled Creatine Kinases in Vivo Regulation of Cellular Respiration-a Synthesis. Mol. Cell. Biochem 1994, 133/134, 155–192. [Google Scholar]
- Saks, VA; Ventura-Clapier, R; Aliev, MK. Metabolic Control and Metabolic Capacity: Two Aspects of Creatine Kinase Functioning in the Cells. Biochim. Biophys. Acta 1996, 1274, 81–92. [Google Scholar]
- Saks, V; Dos Santos, P; Gellerich, FN; Diolez, P. Quantitative Studies of Enzyme-Substrate Compartmentation, Functional Coupling and Metabolic Channelling in Muscle Cells. Mol. Cell. Biochem 1998, 184, 291–307. [Google Scholar]
- Saks, V; Aliev, M; Guzun, R; Beraud, N; Monge, C; Anmann, T; Kuznetsov, AV; Seppet, E. Biophysics of the Organized Metabolic Networks in Muscle and Brain Cells. In Recent Research Developments in Biophysics; Transworld Research Networks: Kerala, India, 2006. [Google Scholar]
- Bessman, SP; Geiger, PJ. Transport of Energy in Muscle: The Phosphorylcreatine Shuttle. Science 1981, 21, 448–452. [Google Scholar]
- Bessman, SP; Carpenter, CL. The Creatine-Creatine Phosphate Energy Shuttle. Annu. Rev. Biochem 1985, 54, 831–862. [Google Scholar]
- Wallimann, T; Wyss, M; Brdiczka, D; Nicolay, K; Eppenberger, HM. Intracellular Compartmentation, Structure and Function of Creatine Kinase Isoenzymes in Tissues with High and Fluctuating Energy Demands: The ‘Phosphocreatine Circuit’ for Cellular Energy Homeostasis. Biochem. J 1992, 281, 21–40. [Google Scholar]
- Dzeja, PP; Vitkevicius, KT; Redfield, MM; Burnett, JC; Terzic, A. Adenylate Kinase-Catalyzed Phosphotransfer in the Myocardium: Increased Contribution in Heart Failure. Circ. Res 1999, 84, 1137–1143. [Google Scholar]
- Dzeja, P; Terzic, A; Wieringa, B. Phosphotransfer Dynamics in Skeletal Muscle from Creatine Kinase Gene-Deleted Mice. Mol Cell Biochem 2004, 256–257, 13–27. [Google Scholar]
- Dzeja, P; Chung, S; Terzic, A. Integration of Adenylate Kinase and Glycolytic and Clycogenolytic Circuits in Cellular Energetics. In Molecular System Bioenergetics Energy for Life; Saks, V, Ed.; Wiley –VCH: Weinheim, Germany, 2007; pp. 265–301. [Google Scholar]
- Saks, VA; Kaambre, T; Sikk, P; Eimre, M; Orlova, E; Paju, K; Piirsoo, A; Appaix, F; Kay, L; Regiz-Zagrosek, V; Fleck, E; Seppet, E. Intracellular Energetic Units in Red Muscle Cells. Biochem. J 2001, 356, 643–665. [Google Scholar]
- Seppet, EK; Kaambre, T; Sikk, P; Tiivel, T; Vija, H; Tonkonogi, M; Sahlin, K; Kay, L; Appaix, F; Braun, U; Eimre, M; Saks, VA. Functional Complexes of Mitochondria with Ca, Mg ATPases of Myofibrils and Sarcoplasmic Reticulum in Muscle Cells. Biochim. Biophys. Acta 2001, 1504, 379–395. [Google Scholar]
- Kaasik, A; Veksler, V; Boehm, E; Novotova, M; Minajeva, A; Ventura-Clapier, R. Energetic Crosstalk between Organelles. Architectural Integration of Energy Production and Utilization. Circ. Res 2001, 89, 153–159. [Google Scholar]
- Wang, SQ; Wei, C; Zhao, G; Brochet, D; Shen, J; Song, LS; Wang, W; Yang, D; Cheng, H. Imaging Microdomain Ca2+ in Muscle Cell. 2004, 94, 1011–1022. [Google Scholar]
- Rizzuto, R; Pozzan, T. Microdomains of Intracellular Ca2+: Molecular Determinants and Functional Consequences. Physiol. Rev 2006, 86, 369–408. [Google Scholar]
- Rostovtseva, TK; Sheldon, KL; Hassanzadeh, E; Monge, C; Saks, V; Bezrukov, SM; Sackett, DL. Tubulin Binding Blocks Mitochondrial Voltage-Dependent Anion Channel and Regulates Respiration. Proc. Natl. Acad. Sci. USA 2008, 105, 18746–18751. [Google Scholar]
- Rostovtseva, TK; Bezrukov, S. VDAC Regulation: Role of Cytosolic Proteins and Mitochondrial Lipids. J. Bioenerg. Biomembr 2008, 40, 163–170. [Google Scholar]
- Monge, C; Beraud, N; Kuznetsov, AV; Rostovtseva, T; Sackett, D; Schlattner, U; Vendelin, M; Saks, VA. Regulation of Respiration in Brain Mitochondria and Synaptosomes: Restrictions of ADP Diffusion in Situ, Roles of Tubulin, and Mitochondrial Creatine Kinase. Mol. Cell. Biochem 2008, 318, 147–165. [Google Scholar]
- Bereiter-Hahn, J. Behaviour of Mitochondria in the Living Cell. Int. Rev. Cytol 1990, 122, 1–63. [Google Scholar]
- Anesti, V; Scorrano, L. The Relationship between Mitochondrial Shape and Function and the Cytoskeleton. Biochim. Biophys. Acta 2006, 1757, 692–699. [Google Scholar]
- Benard, G; Rossignol, R. Ultrastucture of Mitochondria and Its Bearing on Function and Bioenergetics. Antioxidants and Redox Signalling 2008, 10, 1313–1342. [Google Scholar]
- Twig, G; Hyde, B; Shirihai, OS. Mitochondrial Fusion, Fission and Autophagy as a Quality Control Axis: The Bioenergetic View. Biochim. Biophys. Acta 2008, 1777, 1092–1097. [Google Scholar]
- Karbowski, M; Youle, RJ. Dynamics of Mitochondrial Morphology in Healthy Cells and During Apoptosis. Cell Death Differ 2003, 10, 870–880. [Google Scholar]
- Aon, MA; Cortassa, S; Marban, E; O’Rourke, B. Synchronized Whole Cell Oscillations in Mitochondrial Metabolism Triggered by a Local Release of Reactive Oxygen Species in Cardiac Myocytes. J. Biol. Chem 2003, 278, 44735–44744. [Google Scholar]
- Aon, MA; Cortassa, SC; O’Rourke, B. The Fundamental Organization of Cardiac Mitochondria as a Network of Coupled Oscillators. Biophys. J 2006, 91, 4317– 4327. [Google Scholar]
- Collins, TJ; Berridge, MJ; Lipp, P; Bootman, MD. Mitochondria Are Morphologically and Functionally Heterogeneous within Cells. Embo J 2002, 21, 1616–1627. [Google Scholar]
- Pacher, P; Csordás, P; Schneider, T; Hajnóczky, G. Quantification of Calcium Signal Transmission from Sarco-Endoplasmic Reticulum to the Mitochondria. J. Physiol 2000, 529, 553–564. [Google Scholar]
- Zorov, DB; Filburn, CR; Klotz, LO; Zweier, JL; Sollott, SJ. Reactive Oxygen Species (Ros)-Induced Ros Release: A New Phenomenon Accompanying Induction of the Mitochondrial Permeability Transition in Cardiac Myocytes. J. Exp. Med 2000, 192, 1001–1014. [Google Scholar]
- Zorov, DB; Kobrinsky, E; Juhaszova, M; Sollott, SJ. Examining Intracellular Organelle Function Using Fluorescent Probes: From Animalcules to Quantum Dots. Circ. Res 2004, 95, 239–252. [Google Scholar]
- Saks, VA; Kuznetsov, AV; Khuchua, ZA; Vasilyeva, EV; Belikova, JO; Kesvatera, T; Tiivel, T. Control of Cellular Respiration in Vivo by Mitochondrial Outer Membrane and by Creatine Kinase. A New Speculative Hypothesis: Possible Involvement of Mitochondrial-Cytoskeleton Interactions. J. Mol. Cell. Cardiol 1995, 27, 625–645. [Google Scholar]
- Ball, EH; Singer, SJ. Mitochondria Are Associated with Microtubules and Not with Intermediate Filaments in Cultured Fibroblasts. Proc. Natl. Acad. Sci. USA 1982, 79, 123–126. [Google Scholar]
- Capetenaki, Y. Desmin Cytoskeleton: A Potential Regulator of Muscle Mitochondrial Behaviour and Function. Trends Cardiovasc. Med 2002, 12, 339–348. [Google Scholar]
- Vale, RD; Funatsu, T; Pierce, DW; Romberg, L; Harada, Y; Yanagida, T. Direct Observation of Single Kinesin Molecules Moving Along Microtubules. Nature 1996, 380, 451–453. [Google Scholar]
- Vale, RD. The Molecular Motor Toolbox for Intracellular Transport. Cell 2003, 112, 467–480. [Google Scholar]
- Appaix, F; Kuznetsov, AV; Usson, Y; Kay, L; Andrienko, T; Olivares, J; Kaambre, T; Sikk, P; Margreiter, R; Saks, V. Possible Role of Cytoskeleton in Intracellular Arrangement and Regulation of Mitochondria. Exp. Physiology 2003, 88, 175–190. [Google Scholar]
- Milner, DJ; Mavroidis, M; Weisleder, N; Capetanaki, Y. Desmin Cytoskeleton Linked to Muscle Mitochondrial Distribution and Respiratory Function. J. Cell. Biol 2000, 150, 1283–1298. [Google Scholar]
- Anmann, T; Guzun, R; Beraud, N; Pelloux, S; Kuznetsov, AV; Kogerman, L; Kaambre, T; Sikk, P; Paju, K; Peet, N; Seppet, E; Ojeda, C; Tourneur, Y; Saks, V. Different Kinetics of the Regulation of Respiration in Permeabilized Cardiomyocytes and in Hl-1 Cardiac Cells. Importance of Cell Structure/Organization for Respiration Regulation. Biochim. Biophys. Acta 2006, 1757, 1597–1606. [Google Scholar]
- Mannella, CA; Buttle, K; Marko, M. Reconsidering Mitochondrial Structure: New Views of an Old Organelle. Trends Biochem. Sci 1997, 22, 37–38. [Google Scholar]
- Mannella, CA; Pfeiffer, DR; Bradshaw, PC; Moraruv, I; Slepchenko, B; Loew, LM; Hsieh, C; Buttle, K; Marko, M. Topology of the Mitochondrial Inner Membrane: Dynamics and Bioenergetic Implications. IUBMB Life 2001, 52, 93–100. [Google Scholar]
- Mannella, CA; Marko, M; Penczek, P; Barnard, D; Frank, J. The Internal Compartmentation of Rat-Liver Mitochondria: Tomographic Study Using the High-Voltage Transmission Electron Microscope. Microsc. Res. Tech 2001, 27, 278–283. [Google Scholar]
- Mannella, CA. The Relevance of Mitochondrial Membrane Topology to Mitochondrial Function. Biochim. Biophys. Acta 2001, 1762, 140–147. [Google Scholar]
- Aon, MA; Cortassa, S; O’Rourke, B. On the Network Properties of Mitochondria. In Molecular System Bioenergetics, Energy for Life; Saks, V, Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 111–135. [Google Scholar]
- Sun, CN; Dhalla, NS; Olson, RE. Formation of Gigantic Mitochondria in Hypoxic Isolated Perfused Rat Hearts. Experimentia 1969, 25, 763–764. [Google Scholar]
- Lorenz, E; Terzic, A. Physical Association between Recombinant Cardiac ATP-Sensitive K+ Subunits Kir6 and Sur2a. J. Mol. Cell. Cardiol 1999, 31, 425–434. [Google Scholar]
- Crawford, RM; Ranki, HJ; Botting, CH; Budas, GR; Jovanovic, A. Creatine Kinase Is Physically Associated with the Cardiac ATP-Sensitive K+ Channel in Vivo. FASEB J 2002, 16, 102–104. [Google Scholar]
- Carrasco, AJ; Dzeja, PP; Alekseev, AE; Pucar, D; Zingman, LV; Abraham, MR; Hodgson, D; Bienengraeber, M; Puceat, M; Janssen, E; Wieringa, B; Terzic, A. Adenylate Kinase Phosphotransfer Communicates Cellular Energetic Signals to ATP-Sensitive Potassium Channels. Proc. Natl. Acad. Sci. USA 2001, 98, 7623–7628. [Google Scholar]
- Noma, A. ATP-Regulated K+ Channel in Cardiac Muscle. Nature 1983, 305, 147–148. [Google Scholar]
- Noma, A; Shibasaki, T. Membrane Current through Adenosine-Triphosphate Regulated Potassium Channels in Guinea-Pig Ventricular Cells. J. Physiol 1985, 363, 463–480. [Google Scholar]
- Carmeliet, E. A Fuzzy Subsarcolemmal Space for Intracellular Na+ in Cardiac Cells? Cardiovasc. Res 1992, 26, 433–442. [Google Scholar]
- Sasaki, N; Sato, T; Marban, E; O’Rourke, B. ATP Consumption by Uncoupled Mitochondria Activates Sarcolemmal K-ATP Channels in Cardiac Myocytes. Am. J. Physiol 2001, 280, H1882–H1888. [Google Scholar]
- Alekseev, AE; Hodgso, DM; Karger, AB; Park, S; Zingman, LV; Terzic, A. ATP-Sensitive K+ Channel Channel/Enzyme Multimer: Metabolic Gating in the Heart. J. Mol. Cell. Cardiol 2005, 38, 895–905. [Google Scholar]
- Pucar, D; Dzeja, PP; Bast, P; Juranic, N; Macura, S; Terzic, A. Cellular Energetics in the Preconditioned State: Protective Role for Phosphotransfer Reactions Captured by 18O-Assisted 31P NMR. J. Biol. Chem 2001, 276, 44812–44819. [Google Scholar]
- Bers, D. Excitation-Contraction Coupling and Cardiac Contraction; Kluwer Academic Publishers: Dordrecht, Netherlands, 2001. [Google Scholar]
- Bers, D. Cardiac Excitation-Contraction Coupling. Nature 2002, 415, 198–205. [Google Scholar]
- Endoh, M. Signal Transduction and Ca2+ Signaling in Intact Myocardium. J. Pharmacol. Sci 2006, 100, 525–537. [Google Scholar]
- Carafoli, E. Historical Review: Mitochondria and Calcium: Ups and Downs of an Unusual Relationship. Trends Biochem. Sci 2003, 28, 175–181. [Google Scholar]
- Berridge, MJ; Bootman, MD; Roderick, HL. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat. Rev. Mol. Cell. Biol 2003, 4, 517–529. [Google Scholar]
- Rizzuto, R; Bernardi, P; Pozzan, T. Mitochondria as All-Round Players of the Calcium Game. J Physiol 2000, 529, 37–47. [Google Scholar]
- Jacobson, J; Duchen, MR. Interplay between Mitochondria and Cellular Calcium Signalling. Mol Cell Biochem 2004, 256–257, 209–218. [Google Scholar]
- Bianchi, K; Rimessi, A; Prandini, A; Szabadkai, G; Rizzuto, R. Calcium and Mitochondria: Mechanisms and Functions of a Troubled Relationship. Biochim. Biophys. Acta 2004, 1742, 119–131. [Google Scholar]
- Meyer, LE; Machado, LB; Santiago, APSA; da-Silva, S; De Felice, FG; Holub, O; Oliviera, M; Galina, A. Mitochondrial Creatine Kinase Activity Prevents Reactive Oxygen Species Generation: Antioxidant Role of Mitochondrial Kinases-Dependent Adp Re-Cycling Activity. J. Biol. Chem 2006, 281, 37361–37371. [Google Scholar]
- Saks, VA; Favier, R; Guzun, R; Schlattner, U; Wallimann, T. Molecular System Bioenergetics: Regulation of Substrate Supply in Response to Heart Energy Demands. J. Physiol 2006, 577, 769–777. [Google Scholar]
- Williamson, JR. Mitochondrial Function in the Heart. Ann. Rev. Physiol 1979, 41, 485–506. [Google Scholar]
- Neely, JR; Morgan, HE. Relationship between Carbohydrate and Lipid Metabolism and the Energy Balance of Heart Muscle. Annu. Rev. Physiol 1974, 63, 413–459. [Google Scholar]
- Stanley, WC; Recchia, FA; Lopaschuk, GD. Myocardial Substrate Metabolism in the Normal and Failing Heart. Physiol. Rev 2005, 85, 1093–1129. [Google Scholar]
- Aliev, MK; Saks, VA. Compartmentalized Energy Transfer in Cardiomyocytes: Use of Mathematical Modeling for Analysis of in Vivo Regulation of Respiration. Biophys. J 1997, 73, 428–445. [Google Scholar]
- Vendelin, M; Kongas, O; Saks, V. Regulation of Mitochondrial Respiration in Heart Cells Analyzed by Reaction-Diffusion Model of Energy Transfer. Am. J. Physiol. Cell. Physiol 2000, 278, C747–C764. [Google Scholar]
- Cortassa, S; Aon, MA; Marban, E; Winslow, RL; O’Rourke, B. An Integrated Model of Cardiac Mitochondrial Energy Metabolism and Calcium Dynamics. Biophys. J 2003, 84, 2734– 2755. [Google Scholar]
- Cortassa, S; Aon, MA; O’Rourke, B; Jacques, R; Tseng, HJ; Marban, E; Winslow, RL. A Computational Model Integrating Electrophysiology, Contraction, and Mitochondrial Bioenergetics in the Ventricular Myocyte. Biophys. J 2006, 91, 1564–1589. [Google Scholar]
- Beard, DA. Modeling of Oxygen Transport and Cellular Energetics Explains Observations on in Vivo Cardiac Energy Metabolism. PLoS Comput. Biol 2006, 2, 1093–1106. [Google Scholar]
- Matsuoka, S; Sarai, N; Jo, H; Noma, A. Simulation of ATP Metabolism in Cardiac Excitation-Contraction Coupling. Prog. Biophys. Mol. Biol 2004, 85, 279–299. [Google Scholar]
- Korzeniewski, B. Regulation of ATP Supply During Muscle Contraction: Theoretical Studies. Biochem. J 1998, 330, 1189–1195. [Google Scholar]
- Korzeniewski, B; Zoladz, JA. A Model of Oxidative Phosphorylation in Mammalian Skeletal. Muscle. Biophys. Chem 2001, 92, 17–34. [Google Scholar]
- Jafri, MS; Dudycha, SJ; O’Rourke, B. Cardiac Energy Metabolism: Models of Cellular Respiration. Annu. Rev. Biomed. Eng 2001, 3, 57–81. [Google Scholar]
- Saks, VA; Kongas, O; Vendelin, M; Kay, L. Role of the Creatine/Phosphocreatine System in the Regulation of Mitochondrial Respiration. Acta. Physiol. Scand 2000, 168, 635–641. [Google Scholar]
- Barros, LF; Martinez, C. An Enquiry into Metabolite Domains. Biophys. J 2007, 92, 3878–3884. [Google Scholar]
- Seppet, EK; Eimre, M; Andrienko, T; Kaambre, T; Sikk, P; Kuznetsov, AV; Saks, V. Studies of Mitochondrial Respiration in Muscles Cells in Situ: Use and Misuse of Experimental Evidence in Mathematical Modelling. Mol Cell Biochem 2004, 256/257, 219–227. [Google Scholar]
- Wu, F; Yang, F; Vinnakota, KC; Beard, DA. Computer Modelling of Mitochondrial Tricarboxilic Cycle, Oxidative Phosphorylation, Metabolite Transport and Electrophysiology. J. Biol. Chem 2007, 282, 24525–24537. [Google Scholar]
- Wu, F; Zhang, EY; Zhang, J; Bache, RJ; Beard, DA. Phosphate Metabolite Concentrations and ATP Hydrolysis Potential in Normal and Ischemic Hearts. J. Physiol 2008, 586, 4193–4208. [Google Scholar]
- Matthews, P; Taylor, DJ; Radda, GK. Biochemical Mechanisms of Acute Contractile Failure in the Hypoxic Heart. Cardiovasc. Res 1986, 20, 13–19. [Google Scholar]
- Spindler, M; Illing, B; Horn, M; de Groot, M; Ertl, G; Neubauer, S. Temporal Fluctuations of Myocardia High-Energy Phosphate Metabolite with the Cardiac Cycle. Basic. Res. Cardiol 2001, 96, 553–556. [Google Scholar]
- Honda, H; Tanaka, K; Akita, N; Haneda, T. Cyclical Changes in High-Energy Phosphates During the Cardiac Cycle by Pacing-Gated 31p Nuclear Magnetic Resonance. Circ. J 2002, 66, 80–86. [Google Scholar]





© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saks, V.; Monge, C.; Guzun, R. Philosophical Basis and Some Historical Aspects of Systems Biology: From Hegel to Noble - Applications for Bioenergetic Research. Int. J. Mol. Sci. 2009, 10, 1161-1192. https://doi.org/10.3390/ijms10031161
Saks V, Monge C, Guzun R. Philosophical Basis and Some Historical Aspects of Systems Biology: From Hegel to Noble - Applications for Bioenergetic Research. International Journal of Molecular Sciences. 2009; 10(3):1161-1192. https://doi.org/10.3390/ijms10031161
Chicago/Turabian StyleSaks, Valdur, Claire Monge, and Rita Guzun. 2009. "Philosophical Basis and Some Historical Aspects of Systems Biology: From Hegel to Noble - Applications for Bioenergetic Research" International Journal of Molecular Sciences 10, no. 3: 1161-1192. https://doi.org/10.3390/ijms10031161



