Chirality Emergence in Thin Solid Films of Amino Acids by Polarized Light from Synchrotron Radiation and Free Electron Laser
Abstract
:1. Introduction
2. Results and Discussion
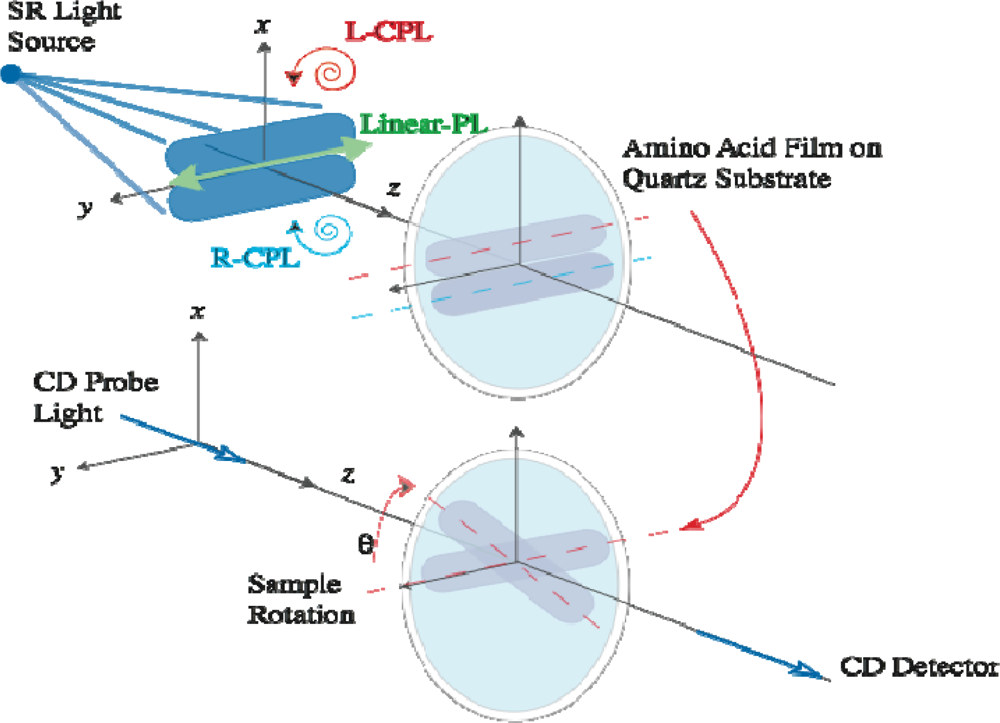
2.1. Polarized Light Irradiation and CD Measurement Apparatus
2.2. Results of LPL Irradiation
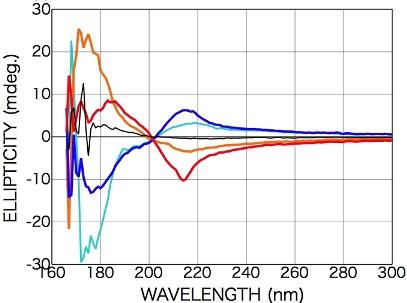
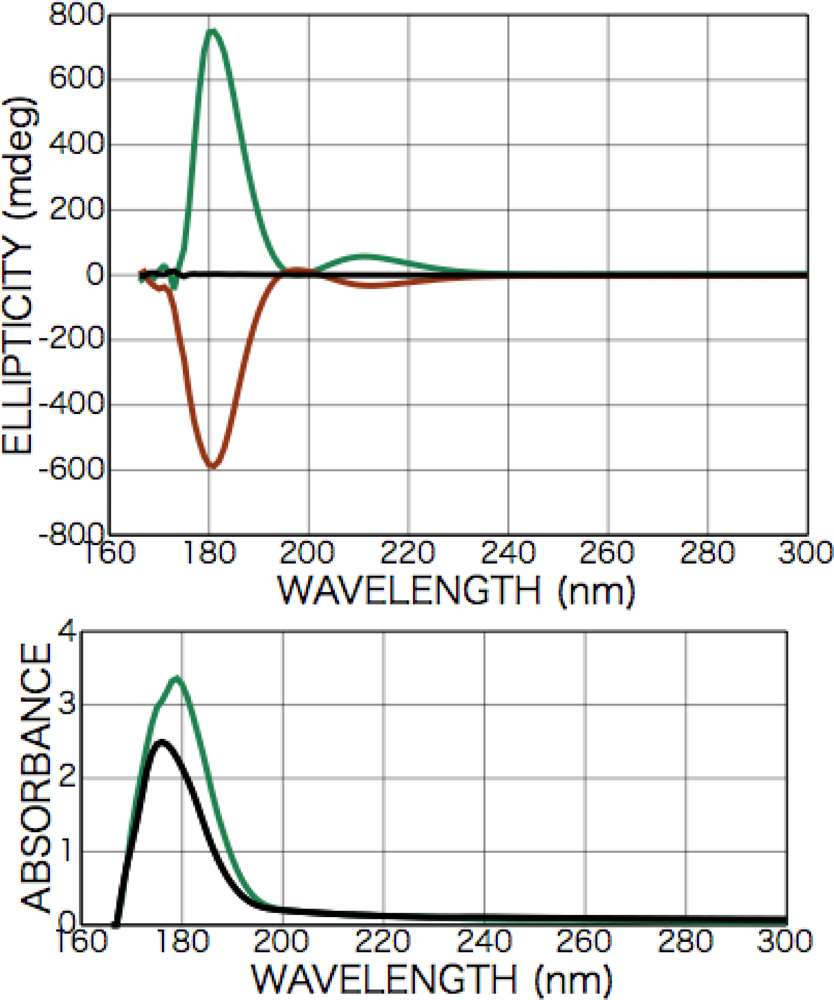
2.2.1. Phenylalanine
| LD, LD’ | linear dichroism in [0° – 90°, 45° – 135°] direction |
| LB, LB’ | linear birefringence in [0°–90°, 45°–135°] direction |
| Px 2, Py 2 | transmittance of photodetector (Px 2 / Py 2 ≅ 1.4) |
| a | optical axis of photodetector (sin 2a ≅ 1) |
| α | residual static birefringence of modulator |
| f1, f2 | quadratic functions of LD, LD’, LB, and LB’ |
2.2.2. Tryptophan
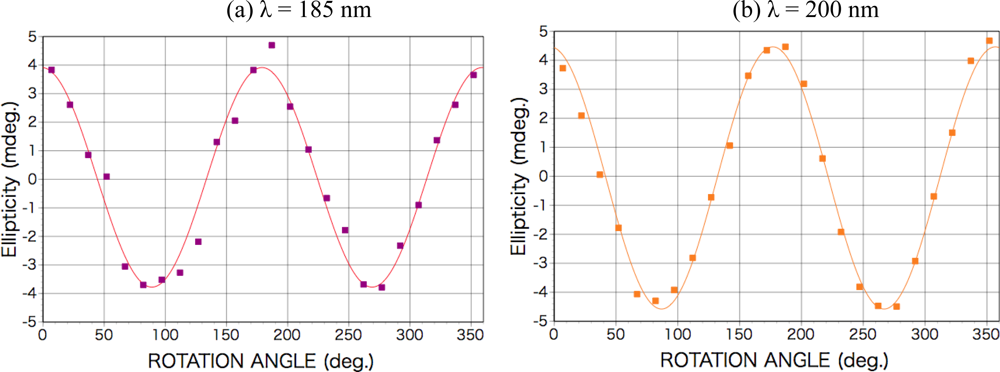
2.3. Results of CPL Irradiation
2.3.1. Phenylalanine
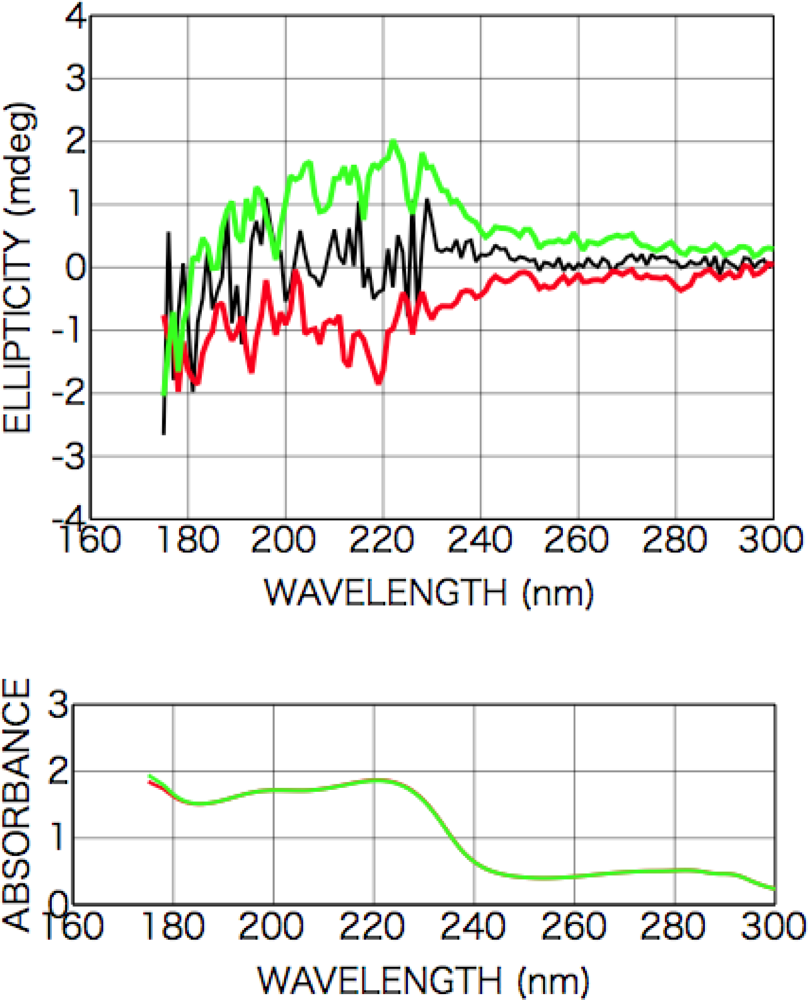
2.3.2. Alanine
2.3.3. Isovaline
2.4. Discussion
3. Experimental Section
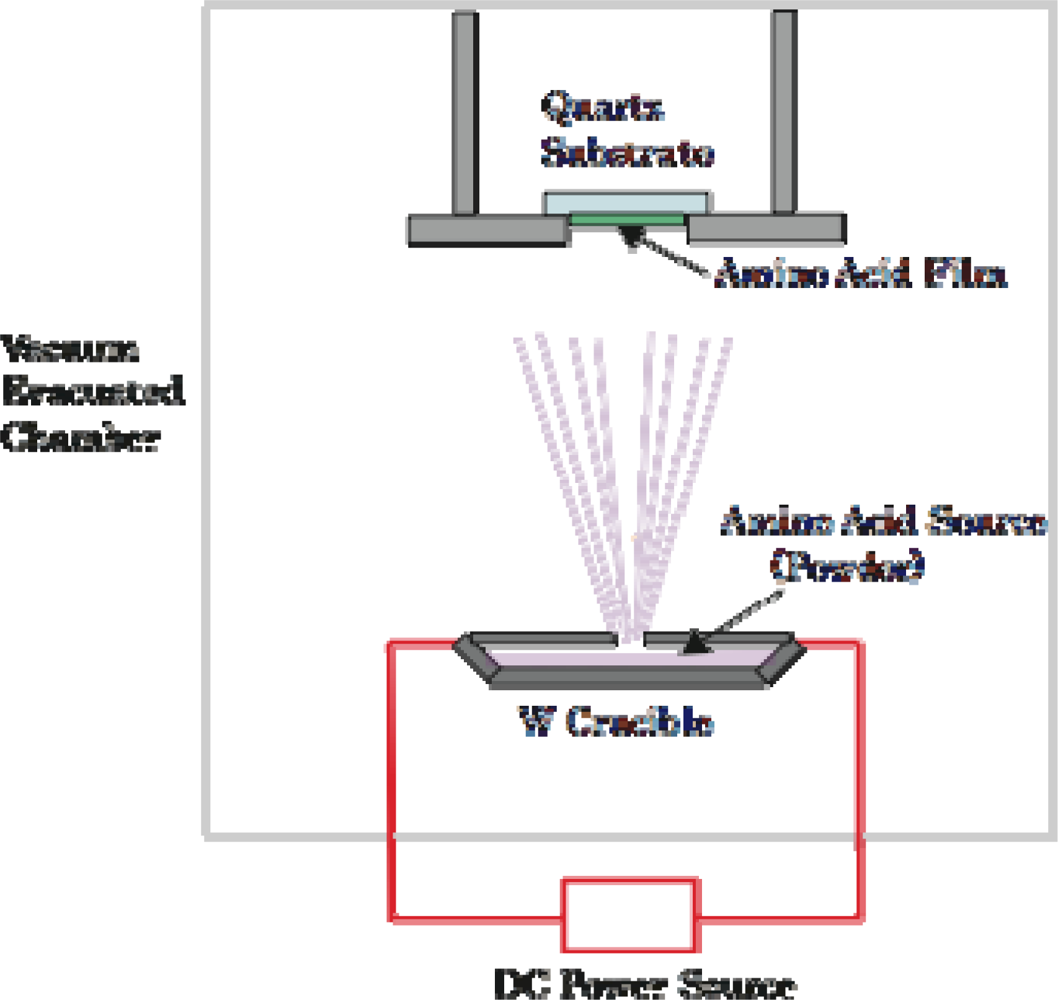
3.1. Amino Acid Film Formation
3.2. Terahertz Absorption Spectroscopy
3.3. Optical Anisotropy Measurements of the Amino Acid Solutions and Films
3.3.1. Phenylalanine
3.3.2. Tryptophan
3.3.3. Alanine
3.3.4. Isovaline
4. Conclusions
Acknowledgments
References and Notes
- Bonner, WA. The origin and amplification of biomolecular chirality. Orig. Life Evol. Biosph 1991, 21, 59–111. [Google Scholar]
- Meierhenrich, UJ. Amino acids and the Asymmetry of Life. In Advances in astrobiology and biogeophysics; Brack, A, Horneck, G, McKay, CP, Stan-Lotter, H, Eds.; Springer-Verlag: Berlin: Heidelberg, Germany, 2008. [Google Scholar]
- Kobayashi, K; Kaneko, K; Takahashi, J; Takano, Y. High molecular weight complex organics in interstellar space and their relevance to origins of life. In Astrobiology: From simple molecules to primitive life; Basiuk, V, Ed.; American Scientific Publisher: Valencia, CA, USA, 2008. [Google Scholar]
- Greenberg, JM; Kouchi, A; Niesson, W; Irth, H; van Paradijs, J; de Groot, M; Hermsen, W. Interstellar dust, chirality, comets, and the origins of life: life from dead stars? J. Biol. Phys 1994, 20, 61–70. [Google Scholar]
- Bailey, J; Chrysostomou, A; Hough, J; Gledhill, T; McCall, A; Clark, S; Menard, F; Tamura, M. Circular polarization in star-formation regions: Implications for biomolecular homochirality. Science 1998, 281, 672–674. [Google Scholar]
- Fukue, T; Tamura, M; Kandori, R; Kusakabe, N; Hough, JH; Lucas, PW; Bailey, J; Whittet, DCB; Nakajima, Y; Hashimoto, J; Nagata, T. Near-infrared circular polarimetry and correlation diagrams in the Orion Becklin-Neugebauer/Kleinman-Low region: Contribution of dichroic extinction. Astrophys. J 2009, 692, L88–L91. [Google Scholar]
- Cline, DB. On the determination of the physical origin of homochirality in life. Comments Nucl. Part. Phys 1996, 22, 131–154. [Google Scholar]
- Tsarev, VA. Neutrino chiral influence from supernova explosion. Kratkie Soobsh Po Fiz 1999, 1, 18–22. [Google Scholar]
- Gusev, GA; Saito, T; Tsarev, VA; Uryson, AV. A Relativistic Neutron Fireball from a Supernova explosion as a possible source of chiral influence. Orig. Life Evol. Biosph 2007, 37, 259–266. [Google Scholar]
- Flores, JJ; Bonner, WA; Massey, GA. Asymmetric photolysis of (RS)-leucine with circularly polarized ultraviolet light. J. Am. Chem. Soc 1977, 99, 3622–3625. [Google Scholar]
- Takano, Y; Kaneko, T; Kobayashi, K; Takahashi, J. Asymmetric photolysis of (DL)-isovaline by synchrotron radiation. Orig. Life Evol. Biosph 2002, 32, 447–448. [Google Scholar]
- Nishino, H; Kosaka, A; Hembury, GA; Shitomi, H; Onuki, H; Inoue, Y. Mechanism of pH-dependent photolysis of aliphatic amino acids and enantiomeric enrichment of racemic leucine by circularly polarized light. Org. Lett 2001, 3, 921–924. [Google Scholar]
- Takano, Y; Takahashi, J; Kaneko, T; Marumo, K; Kobayashi, K. Asymmetric synthesis of amino acid precursors in interstellar complex organics by circularly polarized light. Earth Planet. Sci. Lett 2007, 254, 106–114. [Google Scholar]
- Meierhenrich, UJ; Nahon, L; Alcaraz, C; Bredehöft, JH; Hoffmann, SV; Barbier, B; Brack, A. Asymmetric vacuum UV photolysis of the amino acid leucine in the solid state. Angew. Chem. Int. Ed. Engl 2005, 44, 5630–5634. [Google Scholar]
- Tanaka, M; Kaneko, F; Koketsu, T; Nakagawa, K; Yamada, T. Fragmentation and dimerization of aliphatic amino acid films induced by vacuum ultraviolet irradiation. Rad. Phys. Chem 2008, 77, 1164–1168. [Google Scholar]
- Takahashi, J. Asymmetric photolysis of thin solid film of aromatic amino acid with circularly polarized light. Orig. Life Evol. Biosp 2006, 36, 280–282. [Google Scholar]
- Ehrenfreund, P; Rasmussen, S; Cleaves, J; Chen, L. Experimentally tracing the key steps in the origin of life: The aromatic world. Astrobiology 2006, 6, 490–520. [Google Scholar]
- Cronin, JR; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar]
- Shibayama, A; Kitayama, T; Hayasaka, T; Ido, S; Uno, Y; Hosokawa, T; Nakata, J; Nishimura, K; Nakajima, M. NTT normal-conducting accelerating ring. Rev. Sci. Instr 1989, 60, 1779–1782. [Google Scholar]
- Hosaka, M; Katoh, M; Mochihashi, A; Yamazaki, J; Hayashi, K; Takashima, Y. Upgrade of the UVSOR storage ring FEL. Nucl. Instr. Meth. A 2004, 528, 291–295. [Google Scholar]
- Uno, Y; Hosaka, M. Nagoya University, Nagoya, Japan. Private communication,. 2009.
- Miles, AJ; Wallace, BA. Synchrotron radiation circular dichroism spectroscopy of proteins and application in structural genomics. Chem. Soc. Rev 2006, 35, 39–51. [Google Scholar]
- Sindo, Y; Nishio, M; Maeda, S. Problems of CD spectrometrs (V): Can we measure CD and LD simultaneously? Comments on differential polsarization microscopy (CD and Linear Dichroism). Biopolymers 1990, 30, 405–413. [Google Scholar]
- Kuroda, R; Harada, T; Shindo, Y. A solid-state dedicated circular dichroism spectrophotometer: Development and application. Rev. Sci. Instrum 2001, 72, 3802–3810. [Google Scholar]
- Tanaka, M; Yagi-Watanabe, K; Yamada, T; Kaneko, F; Nakagawa, K. Development of vacuum-ultraviolet circular dichroism measurement system using a polarizing undulator. Chirality 2006, 18, 196–204. [Google Scholar]
- Yamaguchi, M; Miyamaru, F; Yamamoto, K; Tani, M; Hangyo, M. Terahertz absorption spectra of l-, d-, and dl-alanine and their application to determination of enantiometric composition. Appl. Phys. Lett 2005, 86, 053903. [Google Scholar]
- Barth, G; Voelter, W; Mosher, HS; Bunnenberg, E; Djerassi, C. Optical rotatory dispersion studies. CXVII. Absolute configurational assignments of some a-substituted phenylacetic acids by circular dichroism measurements. J. Am. Chem. Soc 1970, 92, 875–886. [Google Scholar]
- Nishino, H; Kosaka, A; Hembury, GA; Matsushima, K; Inoue, Y. The pH dependence of the anisotropy factors of essential amino acids. J Chem Soc Perkin Trans 2002, 2, 582–590. [Google Scholar]
- Auer, HE. Far-ultraviolet absorption and circular dichroism spectra of l-tryptophan and some derivatives. J. Am. Chem. Soc 1973, 95, 3003–3011. [Google Scholar]
- Snyder, PA; Vipond, PM; Johnson, WC, Jr. Circular dichroism of the alkyl amino acids in the vacuum ultraviolet. Biopolymers 1973, 12, 975–992. [Google Scholar]
- Matsuo, K; Matsushima, Y; Fukuyama, T; Senba, S; Gekko, K. Vacuum-ultravilet circular dichroism of amino acids as revealed by synchrotron radiation spectrophotometer. Chem. Lett 2002, 31, 826–827. [Google Scholar]
- Tanaka, M; Kodama, Y; Nakagawa, K. Circular dichroism of amino acid films in UV-VUV region. Enantiomer 2002, 7, 185–190. [Google Scholar]
- Kaneko, F; Yagi-Watanabe, K; Tanaka, M; Nakagawa, K. Natural circular dichroism spectra of alanine and valine films in vacuum ultraviolet region. J. Phys. Soc. Jpn 2009, 78, 013001. [Google Scholar]










© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Takahashi, J.-i.; Shinojima, H.; Seyama, M.; Ueno, Y.; Kaneko, T.; Kobayashi, K.; Mita, H.; Adachi, M.; Hosaka, M.; Katoh, M. Chirality Emergence in Thin Solid Films of Amino Acids by Polarized Light from Synchrotron Radiation and Free Electron Laser. Int. J. Mol. Sci. 2009, 10, 3044-3064. https://doi.org/10.3390/ijms10073044
Takahashi J-i, Shinojima H, Seyama M, Ueno Y, Kaneko T, Kobayashi K, Mita H, Adachi M, Hosaka M, Katoh M. Chirality Emergence in Thin Solid Films of Amino Acids by Polarized Light from Synchrotron Radiation and Free Electron Laser. International Journal of Molecular Sciences. 2009; 10(7):3044-3064. https://doi.org/10.3390/ijms10073044
Chicago/Turabian StyleTakahashi, Jun-ichi, Hiroyuki Shinojima, Michiko Seyama, Yuko Ueno, Takeo Kaneko, Kensei Kobayashi, Hajime Mita, Mashahiro Adachi, Masahito Hosaka, and Masahiro Katoh. 2009. "Chirality Emergence in Thin Solid Films of Amino Acids by Polarized Light from Synchrotron Radiation and Free Electron Laser" International Journal of Molecular Sciences 10, no. 7: 3044-3064. https://doi.org/10.3390/ijms10073044