Effect of Potato Virus Y on the NADP-Malic Enzyme from Nicotiana tabacum L.: mRNA, Expressed Protein and Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Course of Viral Infection Caused by PVYO and by PVYNTN in Nicotiana tabacum L. Plants
2.2. Influence of PVY Infection on NADP-ME Activity
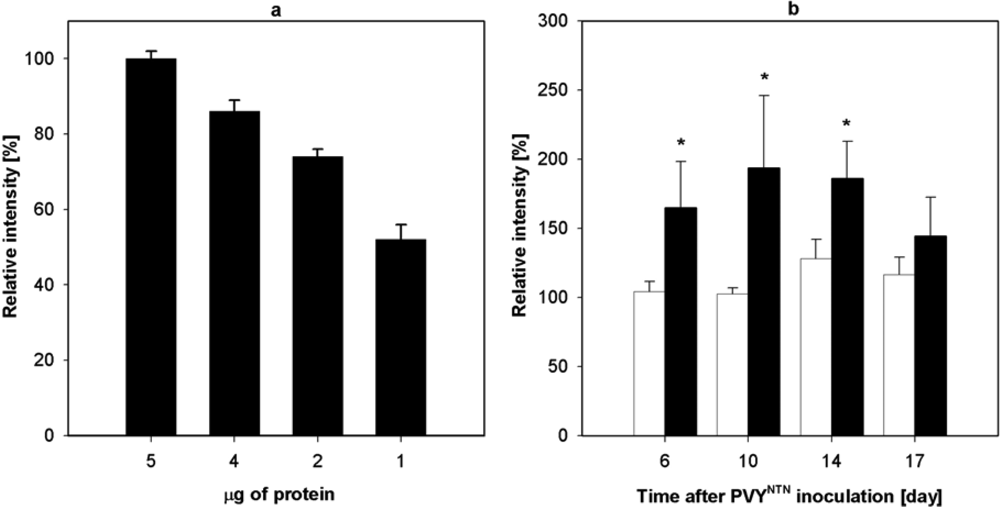
2.3. Influence of PVYNTN Infection on the Expression of NADP-ME
2.4. Influence of PVYNTN Infection on Transcription of NADP-ME
2.5. Proposed Function of NADP-ME during Viral Infection in Plants
3. Experimental Section
3.1. Plant Material
3.2. Enzyme Activity Assay
3.3. Native PAGE
3.4. Purification of NADP-ME from Maize Seeds
3.5. Preparation of Rabbit Antibodies against NADP-ME
3.6. Western Blot Analysis
3.7. mRNA Extraction and Quantification
| MEcyt Forward | ATA CAT TCT TGT TCC TCG GAG CAG |
| MEcyt Reverse | CCC TTT GAA TCC ACC AGC CA |
| MEchl Forward | GCT CTC TTT ATA CAC TGC TCT GG |
| MEchl Reverse | AAG TTC GGC ATA TTC CTG TCC T |
3.8. Metabolite Concentration Measurements
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
References and Notes
- Arias, MC; Lenardon, S; Taleisnik, E. Carbon metabolism alterations in sunflower plants infected with the Sunflower chlorotic mottle virus. J. Phytopathol 2003, 151, 267–273. [Google Scholar]
- Dangl, JL; Jones, JD. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar]
- Hammond-Kosack, KH; Jones, JDG. Responses to plant pathogens. In Biochemistry & Molecular Biology of Plants, 4th ed; Buchanan, BB, Gruissem, W, Jones, RL, Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2002; pp. 1102–1152. [Google Scholar]
- Maurino, VG; Saigo, M; Andreo, CS; Drincovich, MF. Non-photosynthetic ‘malic enzyme’ from maize: A constitutively expressed enzyme that responds to plant defence inducers. Plant Mol. Biol 2001, 45, 409–420. [Google Scholar]
- Synkova, H; Valcke, R. Response to mild water stress in transgenic Pssu-ipt tobacco. Physiol. Plant 2001, 112, 513–523. [Google Scholar]
- Ryslava, H; Muller, K; Semoradova, S; Synkova, H; Cerovska, N. Photosynthesis and activity of phosphoenolpytuvate carboxylase in Nicotiana tabacum L. leaves infected by Potato virus A and Potato virus Y. Photosynthetica 2003, 41, 357–363. [Google Scholar]
- Sun, SB; Shen, QR; Wan, JM; Liu, ZP. Induced expression of the gene for NADP-malic enzyme in leaves of Aloe vera L. under salt stress. Acta Biochim Biophys Sin 2003, 35, 423–429. [Google Scholar]
- Chi, W; Yang, J; Wu, N; Zhang, F. Four rice genes encoding NADP malic enzyme exhibit distinct expression profiles. Biosci Biotechnol, Biochem 2004, 68, 1865–1874. [Google Scholar]
- Synkova, H; Semoradova, S; Burketova, L. High content of endogenous cytokinins stimulates activity of enzymes and proteins involved in stress response in Nicotiana tabacum L. Plant Cell Tiss Org Cult 2004, 79, 169–179. [Google Scholar]
- Smeets, K; Cuypers, A; Lambrechts, A; Semane, B; Hoet, P; van Laere, A; Vangronsveld, J. Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd application. Plant Physiol. Biochem 2005, 43, 437–444. [Google Scholar]
- Edwards, GE; Andreo, CS. NADP-malic enzyme from plants. Phytochemistry 1992, 31, 1845–1857. [Google Scholar]
- Drincovich, MF; Casati, P; Andreo, CS. NADP-malic enzyme from plants: A ubiquitous enzyme involved in different metabolic pathways. FEBS Lett 2001, 490, 1–6. [Google Scholar]
- Chang, HC; Chou, WY; Chang, GG. Effect of metal binding on the structural stability of pigeon liver malic enzyme. J. Biol. Chem 2002, 277, 4663–4671. [Google Scholar]
- Xu, Y; Bhargava, G; Wu, H; Loeber, G; Tong, L. Crystal structure of human mitochondrial NAD(P)+-dependent malic enzyme: A new class of oxidative decarboxylases. Structure 1999, 7, 877–889. [Google Scholar]
- Muller, GL; Drincovich, MF; Andreo, CS; Lara, MV. Nicotiana tabacum NADP-malic enzyme: Cloning, characterization and biological role analysis. Plant Cell Physiol 2008, 49, 469–480. [Google Scholar]
- Bukato, G; Kochan, Z; Swierczynski, J. Purification and properties of cytosolic and mitochondrial malic enzyme isolated from human brain. Int. J. Biochem. Cell Biol 1995, 27, 47–54. [Google Scholar]
- Wheeler, MC; Tronconi, MA; Drincovich, MF; Andreo, CS; Flugge, UI; Maurino, VG. A comprehensive analysis of the NADP-malic enzyme gene family of Arabidopsis. Plant Physiol 2005, 139, 39–51. [Google Scholar]
- Tausta, SL; Coyle, HM; Rothermel, B; Stiefel, V; Nelson, T. Maize C4 and non-C4 NADP-dependent malic enzymes are encoded by distinct genes derived from a plastid-localized ancestor. Plant Mol. Biol 2002, 50, 635–652. [Google Scholar]
- Wheeler, MC; Arias, CL; Tronconi, MA; Maurino, VG; Andreo, CS; Drincovich, MF. Arabidopsis thaliana NADP-malic enzyme isoforms: High degree of identity but clearly distinct properties. Plant Mol. Biol 2008, 67, 231–242. [Google Scholar]
- Cerovska, N. Production of monoclonal antibodies to potato virus YNTN strain and their use for strain differentiation. Plant Pathol 1998, 47, 505–509. [Google Scholar]
- Synkova, H; Semoradova, S; Schnablova, R; Muller, K; Pospisilova, J; Ryslava, H; Malbeck, J; Cerovska, N. Effects of biotic stress caused by Potato virus Y on photosynthesis in ipt transgenic and control Nicotiana tabacum L. Plant Sci 2006, 171, 607–616. [Google Scholar]
- Doubnerova, V; Jiraskova, A; Janoskova, M; Muller, K; Bat'kova, P; Synkova, H; Cerovska, N; Ryslava, H. The activity and isoforms of NADP-malic enzyme in Nicotiana benthamiana plants under biotic stress. Gen. Physiol. Biophys 2007, 26, 281–289. [Google Scholar]
- Saher, S; Fernandez-Garcia, N; Piqueras, A; Hellin, E; Olmos, E. Reducing properties, energy efficiency and carbohydrate metabolism in hyperhydric and normal carnation shoots cultured in vitro: A hypoxia stress? Plant Physiol. Biochem 2005, 43, 573–582. [Google Scholar]
- Casati, P; Lara, MV; Andreo, CS. Induction of a C(4)-like mechanism of CO(2) fixation in Egeria densa, a submersed aquatic species. Plant Physiol 2000, 123, 1611–1622. [Google Scholar]
- Crecelius, F; Streb, P; Feierabend, J. Malate metabolism and reactions of oxidoreduction in cold-hardened winter rye (Secale cereale L.) leaves. J. Exp. Bot 2003, 54, 1075–1083. [Google Scholar]
- Valderrama, R; Corpas, FJ; Carreras, A; Gomez-Rodriguez, MV; Chaki, M; Pedrajas, JR; Fernandez-Ocana, A; Del Rio, LA; Barroso, JB. The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ 2006, 29, 1449–1459. [Google Scholar]
- Liu, S; Cheng, Y; Zhang, X; Guan, Q; Nishiuchi, S; Hase, K; Takano, T. Expression of an NADP-malic enzyme gene in rice (Oryza sativa L.) is induced by environmental stresses; over-expression of the gene in Arabidopsis confers salt and osmotic stress tolerance. Plant Mol. Biol 2007, 64, 49–58. [Google Scholar]
- Ryslava, H; Doubnerova, V; Muller, K; Bat’kova, P; Schnablova, R; Liberda, J; Synkova, H; Cerovska, N. The enzyme kinetics of the NADP-malic enzyme from tobacco leaves. Collect. Czech. Chem. Commun 2007, 72, 1420–1434. [Google Scholar]
- Rizhsky, L; Hongjian, L; Mittler, R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 2002, 130, 1143–1151. [Google Scholar]
- Lara, MV; Drincovich, MF; Muller, GL; Maurino, VG; Andreo, CS. NADP-malic enzyme and Hsp70: Co-purification of both proteins and modification of NADP-malic enzyme properties by association with Hsp70. Plant Cell Physiol 2005, 46, 997–1006. [Google Scholar]
- Wang, W; Vinocur, B; Shoseyov, O; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 2004, 9, 244–252. [Google Scholar]
- Casati, P; Drincovich, MF; Edwards, GE; Andreo, CS. Malate metabolism by NADP-malic enzyme in plant defense. Photosynth Res 1999, 61, 99–105. [Google Scholar]
- Muller, K; Doubnerova, V; Synkova, H; Cerovska, N; Ryslava, H. Regulation of phosphoenolpyruvate carboxylase in PVY(NTN)-infected tobacco plants. Biol. Chem 2008, 390, 245–251. [Google Scholar]
- Edwards, GE; Franceschi, VR; Voznesenskaya, EV. Single-cell C(4) photosynthesis versus the dual-cell (Kranz) paradigm. Annu. Rev. Plant Biol 2004, 55, 173–196. [Google Scholar]
- Hibberd, JM; Quick, WP. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 2002, 415, 451–454. [Google Scholar]
- Clark, MF; Adams, AN. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol 1977, 34, 475–483. [Google Scholar]
- Lee, DH; Lee, CB. Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: In gel enzyme activity assays. Plant Sci 2000, 159, 75–85. [Google Scholar]
- Ferguson, KA. Starch-gel electrophoresis—Application to the classification of pituitary proteins and polypeptides. Metabolism 1964, 13, 985–1002. [Google Scholar]
- Steinbuch, M; Audran, R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys 1969, 34, 279–284. [Google Scholar]
- Laemmli, UK. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 1970, 227, 680–685. [Google Scholar]
- Stitt, M; Lilley, RM; Gerhardt, R; Heldt, HW. Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol 1989, 174, 518–552. [Google Scholar]






© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Doubnerová, V.; Müller, K.; Čeřovská, N.; Synková, H.; Spoustová, P.; Ryšlavá, H. Effect of Potato Virus Y on the NADP-Malic Enzyme from Nicotiana tabacum L.: mRNA, Expressed Protein and Activity. Int. J. Mol. Sci. 2009, 10, 3583-3598. https://doi.org/10.3390/ijms10083583
Doubnerová V, Müller K, Čeřovská N, Synková H, Spoustová P, Ryšlavá H. Effect of Potato Virus Y on the NADP-Malic Enzyme from Nicotiana tabacum L.: mRNA, Expressed Protein and Activity. International Journal of Molecular Sciences. 2009; 10(8):3583-3598. https://doi.org/10.3390/ijms10083583
Chicago/Turabian StyleDoubnerová, Veronika, Karel Müller, Noemi Čeřovská, Helena Synková, Petra Spoustová, and Helena Ryšlavá. 2009. "Effect of Potato Virus Y on the NADP-Malic Enzyme from Nicotiana tabacum L.: mRNA, Expressed Protein and Activity" International Journal of Molecular Sciences 10, no. 8: 3583-3598. https://doi.org/10.3390/ijms10083583





