Eu-Doped BaTiO3 Powder and Film from Sol-Gel Process with Polyvinylpyrrolidone Additive
Abstract
:1. Introduction
2. Results and Discussion
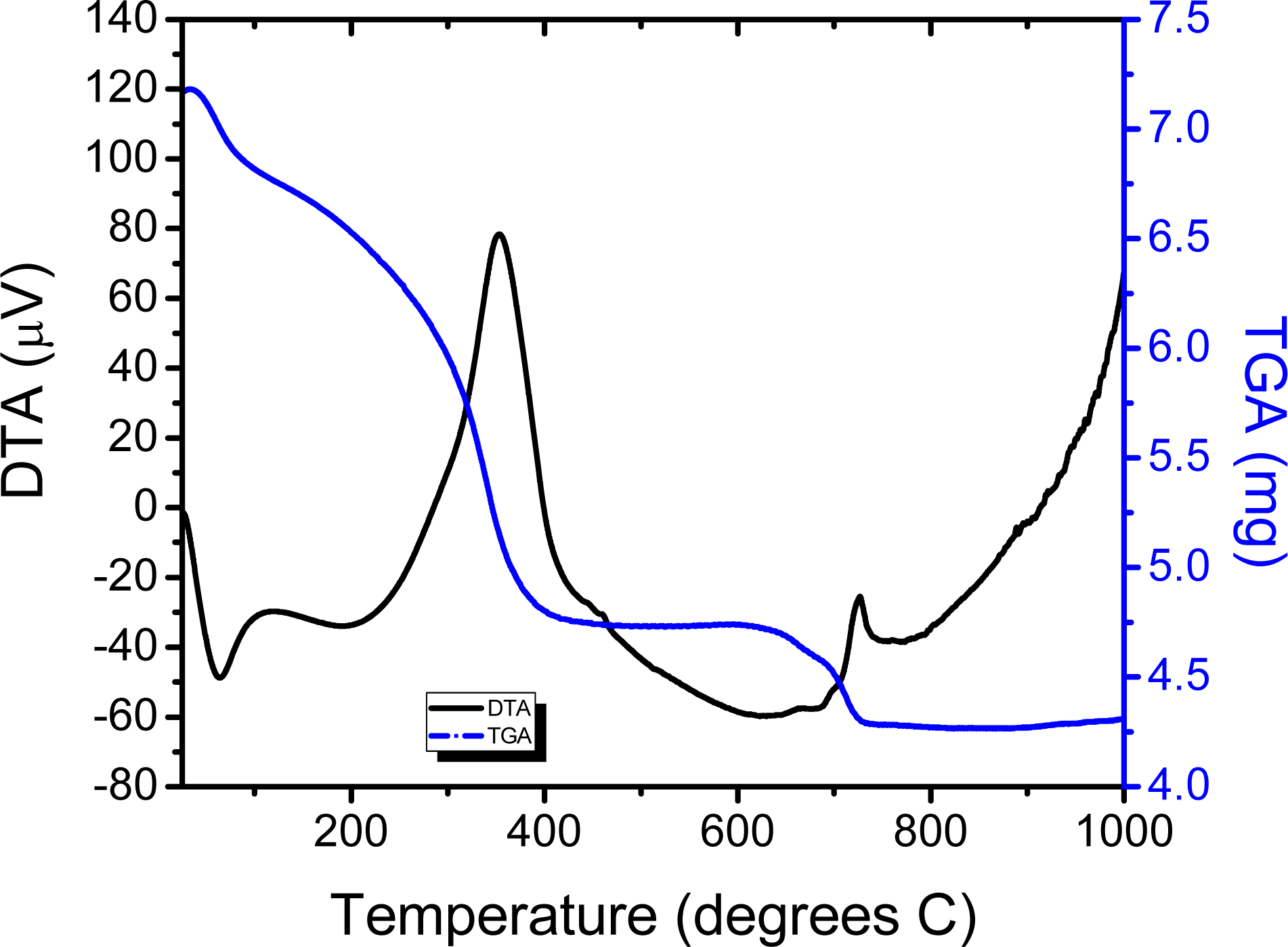
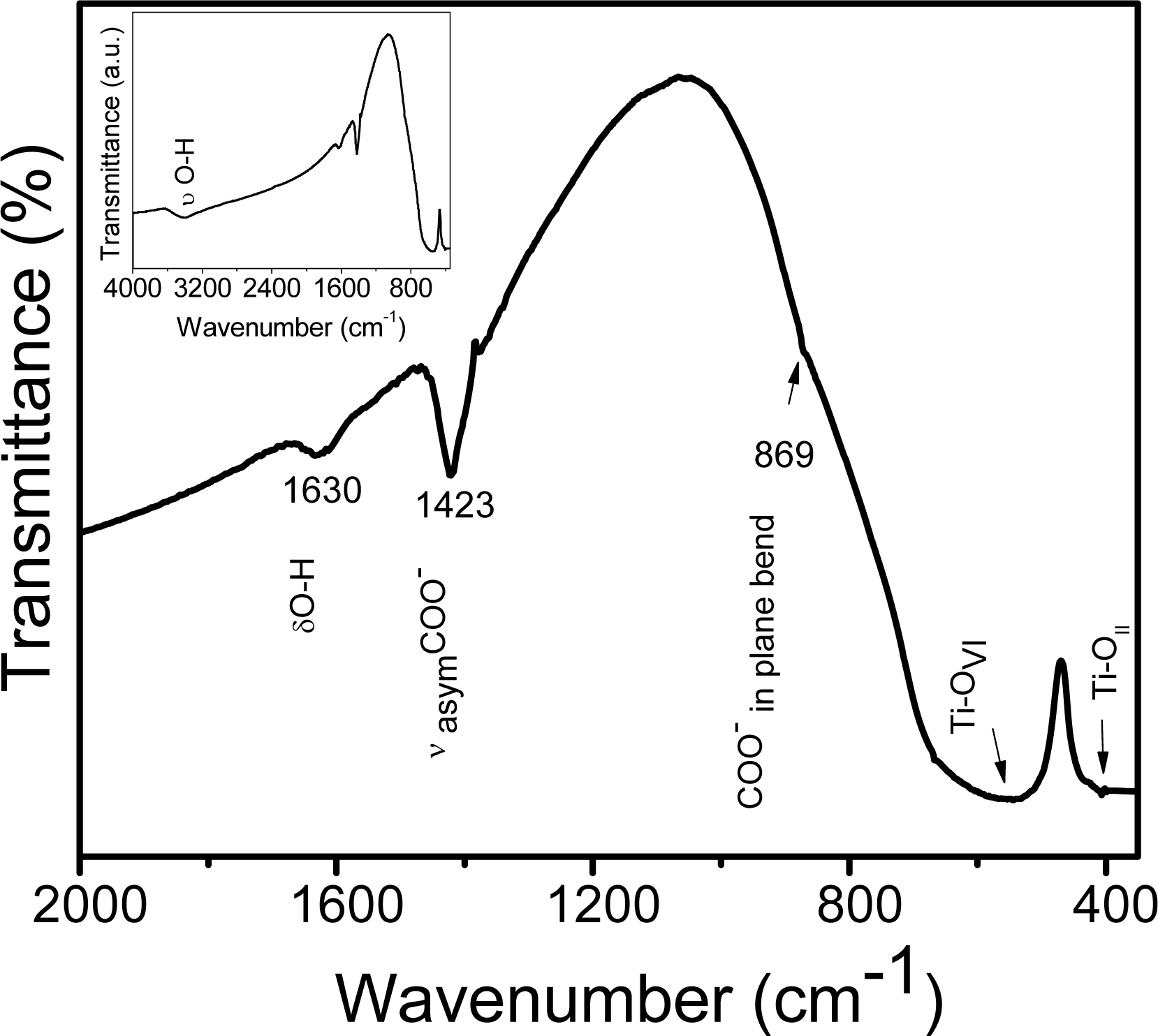
2.1. Chemical Studies
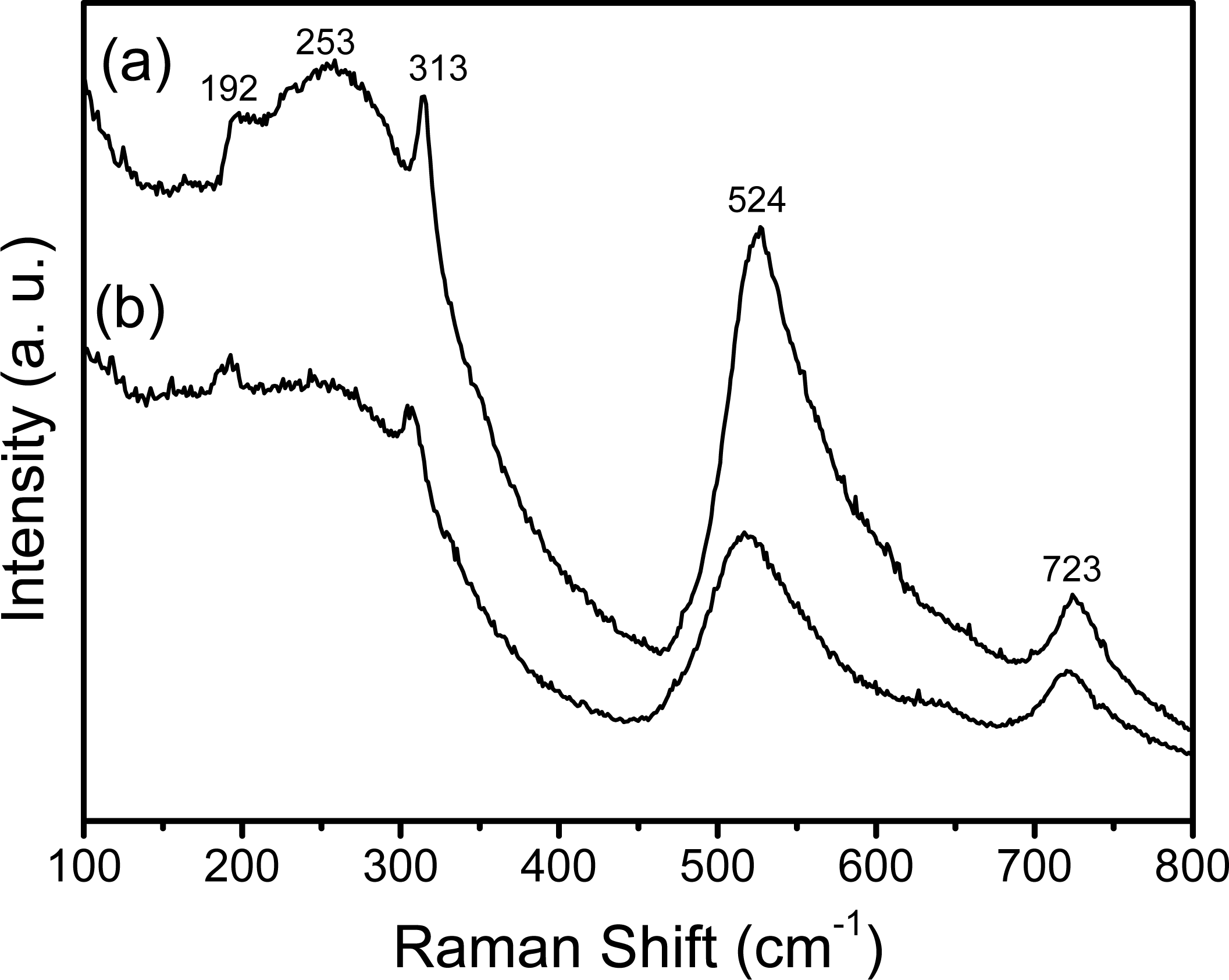
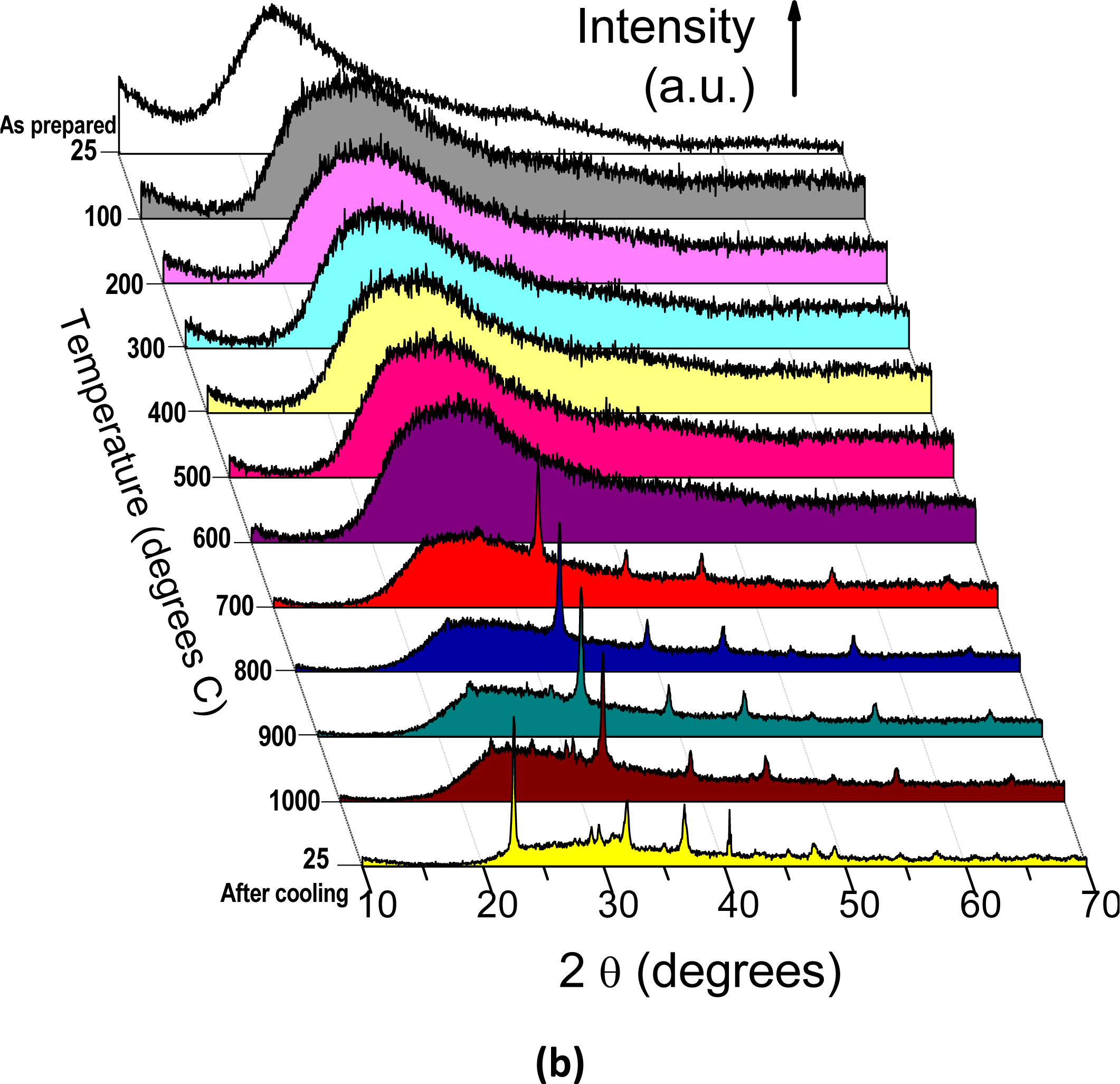
2.2. HT-XRD and XRD Studies
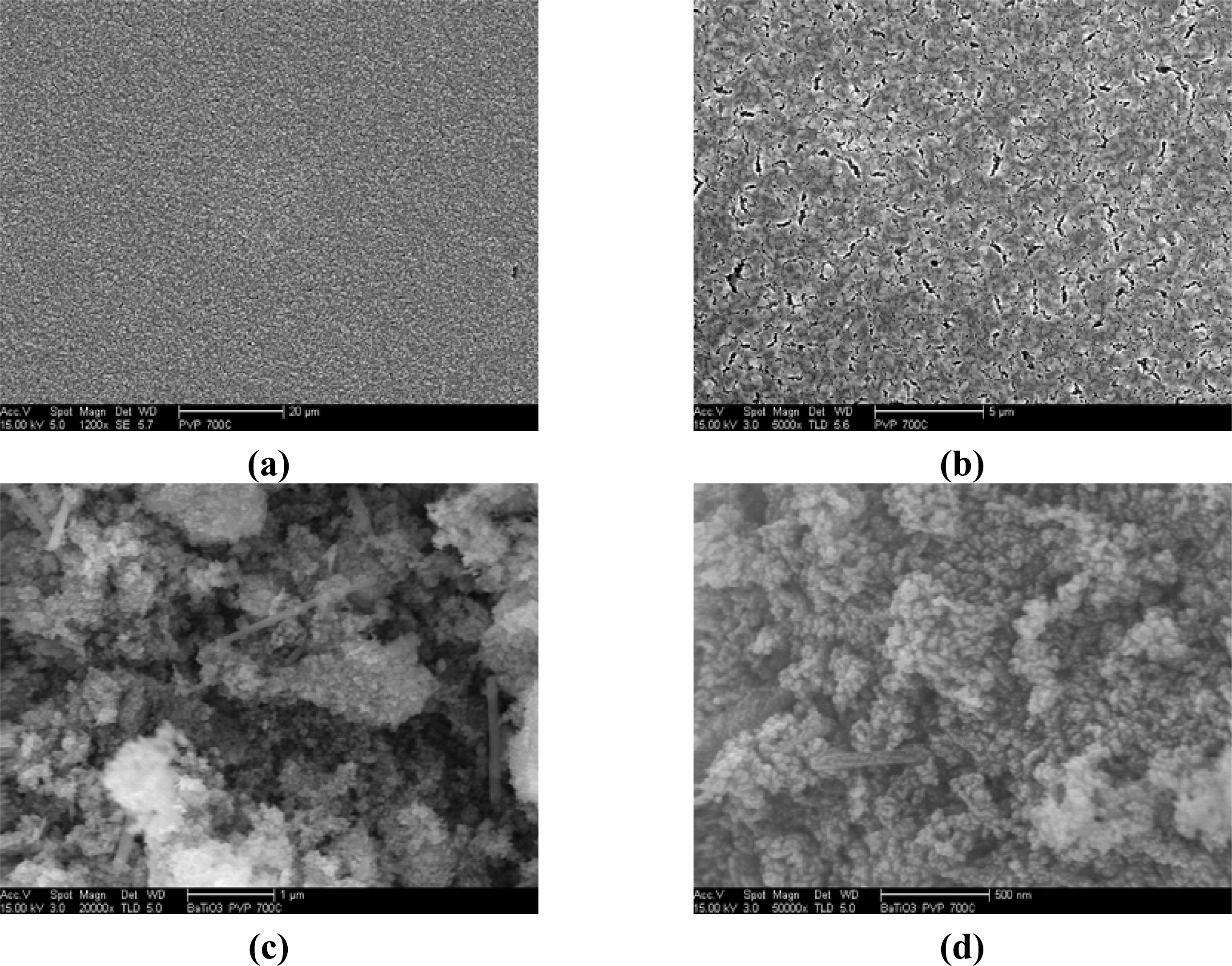
2.3. Microscopy Observations
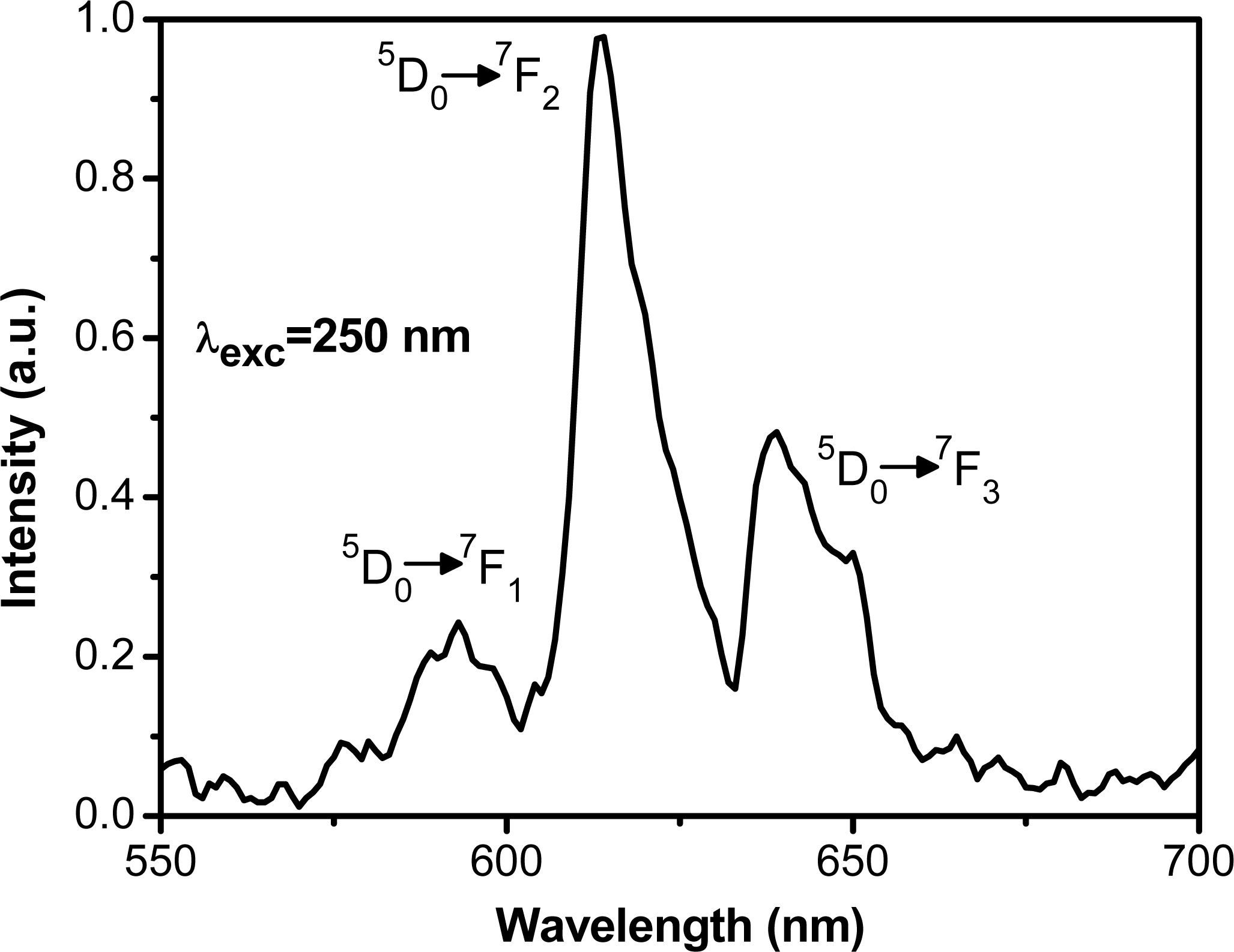
2.4. Luminescence Properties
3. Experimental Section
3.1. Experimental Procedure
3.2. Apparatus
4. Conclusions
Acknowledgments
References and notes
- Battisha, IK; Beyally, AEl; El Mongy, SA; Nahrawi, AM. Development of the FTIR properties of nano-structure silica gel doped with different rare earth elements, prepared by sol-gel route. J. Sol-Gel Sci. Technol 2007, 41, 129–137. [Google Scholar]
- Battisha, K; El Beyally, A; Soliman, SL; El Nahrawi, AS. Indian, structural and optical studies of activated thin film and monolith nano-structure silica gel with different rare earth elements prepared by sol-gel techniques. Indian J. Pure Appl. Phys 2007, 45, 441–453. [Google Scholar]
- Tissue, BM. Synthesis and luminescence of lanthanide ions in nanoscale insulating hosts. Chem. Mater 1998, 10, 2837–2845. [Google Scholar]
- Zhou, J; Li, L; Gui, Z; Buddhudu, S; Zhou, Y. Photoluminescence of CdSe nanocrystallites embedded in BaTiO3 matrix. Appl. Phys. Lett 2000, 76, 1540–1542. [Google Scholar]
- Huang, CH; McCaughan, L. Polarization-dependent enhancement of population inversion and of green upconversion in Er:LiNbO3 by Yb codoping. IEEE Photonics Technol. Lett 1997, 9, 599–601. [Google Scholar]
- Itoh, S; Toki, H; Tamura, K; Kataoka, F. A new red-emitting phosphor, SrTiO3:Pr3+, for low-voltage electron excitation. J. Appl. Phys 1999, 38, 6387–6391. [Google Scholar]
- Allak, HM; Brinkman, AW; Russell, GJ; Woods, J. The effect of Mn on the positive temperature coefficient of resistance characteristics of donor doped BaTiO3 ceramics. J. Appl. Phys 1988, 63, 4530–4535. [Google Scholar]
- Morales Ramírez, AdeJ; García Murillo, A; Carrillo Romo, FdJ; Ramírez Salgado, J; Le Luyer, C; Chadeyron, G; Boyer, D; Moreno Palmerin, J. Preparation and studies of Eu3+ and Tb3+ co-doped Gd2O3 and Y2O3 sol–gel scintillating films. Thin Solid Films 2009. [Google Scholar]
- García Murillo, A; de J. Carrillo Romo, F; Le Luyer, C; de J. Morales Ramirez, A; García Hernández, M; Moreno Palmerin, J. Sol–gel elaboration and structural investigations of Lu2O3:Eu3+ planar waveguides. J. Sol-Gel Sci. Technol 2009, 50, 359–367. [Google Scholar]
- Hreniak, D; Strek, W; Amami, J; Guyot, Y; Boulon, G; Goutaudier, C; Pazik, R. The size-effect on luminescence properties of BaTiO3:Eu3+ nanocrystallites prepared by the sol–gel method. J. Alloys Comp 2004, 380, 348–351. [Google Scholar]
- Badr, Y; Salah, A; Battisha, I. Effect of europium ion concentrations on the photoluminescence emission of nano-crystalline BaTiO3 prepared by sol–gel technique. J. Sol-Gel Sci. Technol 2005, 34, 219–226. [Google Scholar]
- Hreniak, D; Strek, W; Chmielowiec, J; Pasciak, G; Pazik, R; Gierlotka, S; Lojkowski, W. Preparation and conductivity measurement of Eu doped BaTiO3 nanoceramic. J Alloys Comp 2006, 408, 412, 637–640. [Google Scholar]
- Pazik, R; Hreniak, D; Strek, W; Kessler, VG; Seisenbaeva, GA. Photoluminescence investigations of Eu3+ doped BaTiO3 nanopowders fabricated using heterometallic tetranuclear alkoxide complexes. J. Alloys Comp 2008, 451, 557–562. [Google Scholar]
- Rath, MK; Pradhan, GK; Pandey, B; Verma, HC; Roul, BK; Anand, S. Synthesis, characterization and dielectric properties of europium-doped barium titanate nanopowders. Mat. Lett 2008, 62, 2136–2139. [Google Scholar]
- Li, J; Kuwabara, M. Preparation and luminescent properties of Eu-doped BaTiO3 thin films by sol–gel process. Sci. Technol. Adv. Mat 2003, 4, 143–148. [Google Scholar]
- Hoerman, H; Ford, GM; Kaufmann, LD; Wessels, BW. Dynamic response of the dielectric and electro-optic properties of epitaxial ferroelectric thin films. J. Appl. Phys. Lett 1998, 73, 2248–2250. [Google Scholar]
- Yoon, SG; Safari, A. (Ba0,5, Sr0.5)TiO3 thin film preparation by r.f. magnetron sputtering and its electric properties. Thin Solid Films 1995, 254, 211–215. [Google Scholar]
- Li, J; Wu, YJ; Yamamoto, T; Kuwabara, M. Electrophoretic deposition and photoluminescent properties of Eu-doped BaTiO3 thin film from a suspension of monodispersed nanocrystallites. Sci. Technol. Adv. Mat 2004, 5, 393–398. [Google Scholar]
- Battisha, IK; Speghini, A; Polizzi, S; Agnoli, F; Bettinelli, M. Molten chloride synthesis, structural characterisation and luminescence spectroscopy of nanocrystalline Eu3+ doped BaTiO3 and SrTiO3. Mat. Lett 2002, 57, 183–187. [Google Scholar]
- Xu, J; Zhai, J; Yao, X; Xue, J; Huang, Z. Dielectric and optical properties of BaTiO3 thin films prepared by low-temperature process. J. Sol-Gel Sci. Technol 2007, 42, 209–212. [Google Scholar]
- Matsuda, H; Kobayashi, N; Kobayashi, T; Miyazaya, K; Kuwabara, M. Room-temperature synthesis of crystalline barium titanate thin films by high–concentration sol–gel method. J. Non-Cryst. Sol 2000, 271, 162–166. [Google Scholar]
- Manso-Silván, M; Fuentes-Cobas, L; Martín-Palma, RJ; Hernández-Vélez, M; Martínez-Duart, JM. BaTiO3 thin films obtained by sol–gel spin coating. Surf Coat Technol 2002, 151, 152, 118–121. [Google Scholar]
- Qifang, L; Dairong, C; Xiuling, J. Preparation and characterization of BaTiO3 long fibers by sol-gel process using catechol-complexed alkoxide. J. Sol-Gel Sci. Technol 2002, 25, 243–248. [Google Scholar]
- Shimooka, H; Ken-ichi, Y; Seiji, T. Preparation of transparent, partially-crystallized BaTiO3 monolithic xerogels by sol-gel processing. J. Sol-Gel Sci. Technol 1998, 13, 873–876. [Google Scholar]
- Sharma, HB; Mansingh, A. Sol-gel processed barium titanate ceramics and thin films. J. Mater. Sci 1998, 33, 4455–4459. [Google Scholar]
- Henry, M; Jolivet, JP; Livage, J. Structure and Bonding; Springer-Verlag Berlin Heidelberg: New York, NY, USA, 1992. [Google Scholar]
- Henry, M; Jolivet, JP; Livage, J. Ultrastructure Processing of Advance Materials; John Wiley & Sons, Inc: Hoboken, NJ, USA, 1992. [Google Scholar]
- Doeuff, S; Henry, M; Sanchez, C; Livage, J. Hydrolysis of titanium alkoxides: Modification of the molecular precursor by acetic acid. J. Non-Cryst. Solids 1987, 89, 206–216. [Google Scholar]
- Mosset, A; Luneau, I; Galy, J. Sol-gel processed BaTiO3: Structural evolution from the gel to the crystallisation powder. J Non-Cryst Solids 1988, 100, 339–344. [Google Scholar]
- Bernier, JC; Rehspringer, JL; Vilminot, S; Poix, P. Synthesis and sintering comparison of cordierite powders. Mat. Res. Soc. Symp. Proc 1986, 73, 129–134. [Google Scholar]
- Kozuka, H; Takenaka, S; Tokita, H; Okubayashi, M. PVP-assisted sol-gel deposition of single layer ferroelectric thin films over submicron or micron in thickness. J. Eur. Ceram. Soc 2004, 24, 1585–1588. [Google Scholar]
- Kozuka, H; Kajimura, M; Hirano, T; Katayama, K. Crack-free thick ceramic coating films via non-repetitive dip-coating using polyvinylpyrrolidone as stress-relaxing agent. J. Sol-Gel Sci. Technol 2000, 19, 205–209. [Google Scholar]
- Kozuka, H; Higuchi, A. Stabilization of poly(vinylpyrrolidone)-containing alkoxide solutions for thick sol-gel barium titanate films. J. Am. Ceram. Soc 2003, 86, 33–38. [Google Scholar]
- Madarasz, J; Kaneko, S; Okuya, M; Pokol, G. Comparative evolved gas analyses of crystalline and amorphous titanium(IV)oxo-hydroxo-acetylacetonates by TG-FTIR and TG/DTA-MS. Thermochim. Acta 2009, 489, 37–44. [Google Scholar]
- Legrand-Buscema, C; Malibert, C; Bach, S. Elaboration and characterization of thin films of TiO2 prepared by sol-gel process. Thin Sol. Films 2002, 418, 79–84. [Google Scholar]
- Watanabe, K; Ohsato, H; Kishi, H; Okino, Y; Kohzu, N; Iguchi, Y; Okuda, T. Solubility of La–Mg and La–Al in BaTiO3. Solid State Ionics 1998, 108, 129–135. [Google Scholar]
- Harizanov, OA. Sol-gel BaTiO3 from a peptized solution. Mat. Lett 1998, 34, 232–236. [Google Scholar]
- Ghosh, S; Dasgupta, S; Sen, A; Maiti, HS. Synthesis of barium titanate nanopowder by a soft chemical process. Mat. Lett 2007, 61, 538–541. [Google Scholar]
- Amami, J; Hreniak, D; Guyot, Y; Pazik, R; Goutaudier, C; Boulon, G; Ayadi, M; Strek, W. Second harmonic generation and Yb3+ cooperative emission used as structural probes in size-driven cubic–tetragonal phase transition in BaTiO3 sol–gel nanocrystals. J Lumin 2006, 119, 120, 383–387. [Google Scholar]
- Zhu, X; Wang, J; Zhang, Z; Zhu, J; Zhou, S; Liu, Z; Ming, N. Atomic-scale characterization of barium titanate powders formed by the hydrothermal process. J. Am. Ceram. Soc 2008, 91, 1002–1008. [Google Scholar]
- An, C; Liu, C; Wang, S; Liu, Y. Generalized large-scale synthesis of MTiO3 (M = Ba, Sr, Pb) nanocrystals. Mat. Res. Bull 2008, 43, 932–938. [Google Scholar]
- Song-Wei, L; Burtrand, IL; Zhong-Lin, W; William, DS. Hydrothermal synthesis and structural characterization of BaTiO3 nanocrystals. J. Crystal Growth 2000, 219, 269–276. [Google Scholar]
- Asiaie, R; Zhu, W; Akbar, SA; Dutta, PK. Characterization of submicron particles of tetragonal BaTiO3. Chem. Mater 1996, 8, 226–234. [Google Scholar]
- Amami, J; Hreniak, D; Guyot, Y; Pazik, R; Strek, W; Goutaudier, C; Boulon, G. New optical tools used for characterization of phase transitions in nonlinear nano-crystals. Example of Yb3+-doped BaTiO3. J. Phys. Condens. Matter 2007, 19, 1–14. [Google Scholar]
- Ma, WH; Zhang, MS; Yin, Z. Phonon characteristics of thin film and nanophase lead titanate. J. Korean Phys. Soc 1998, 32, 1137–1139. [Google Scholar]
- García-Murillo, A; Carrillo-Romo, FJ; García-Hernández, M; Barbosa-García, O; Meneses-Nava, A; Palomares-Sánchez, S; Flores-Vela, A. Structural and optical characteristics of BaTiO3:Yb3+ powders. Mat Trans 2009, 50, 1850–1854. [Google Scholar]
- Wein-Duo, Y; Haile, SM. Highly preferred oriented lead barium titanate thin films using acetylacetone as chelating agent in a sol-gel process. Rev. Adv. Mater. Sci 2005, 10, 143–148. [Google Scholar]
- Kozuka, H; Takenaka, S. Single-step deposition of gel-derived lead zirconate titanate films: Critical thickness and gel film to ceramic film conversion. J. Am. Ceram. Soc 2002, 85, 2696–2702. [Google Scholar]
- Gary, SM; Costas, JS. Fundamentals of Semiconductor Manufacturing and Process Control; Wiley-Interscience, A John wiley & Sons, Inc: Hoboken, NJ, USA, 2006; p. 63. [Google Scholar]
- Hreniak, D; Strek, W; Amami, J; Guyot, Y; Boulon, G; Goutaudier, C; Pazik, R. The size-effect on luminescence properties of BaTiO3:Eu3+ nanocrystallites prepared by the sol–gel method. J. Alloys Comp 2004, 380, 348–351. [Google Scholar]
- Pązik, R; Hreniak, D; Stręk, W; Kessler, VG; Seisenbaeva, GA. Photoluminescence investigations of Eu3+ doped BaTiO3 nanopowders fabricated using heterometallic tetranuclear alkoxide complexes. J. Alloys Comp 2008, 451, 557–562. [Google Scholar]
- Blasse, G; Grabmaier, BC. Luminescent Materials; Springer-Verlag: New York, NY, USA, 1994. [Google Scholar]



© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
García-Hernández, M.; García-Murillo, A.; Carrillo-Romo, F.d.J.; Jaramillo-Vigueras, D.; Chadeyron, G.; De la Rosa, E.; Boyer, D. Eu-Doped BaTiO3 Powder and Film from Sol-Gel Process with Polyvinylpyrrolidone Additive. Int. J. Mol. Sci. 2009, 10, 4088-4101. https://doi.org/10.3390/ijms10094088
García-Hernández M, García-Murillo A, Carrillo-Romo FdJ, Jaramillo-Vigueras D, Chadeyron G, De la Rosa E, Boyer D. Eu-Doped BaTiO3 Powder and Film from Sol-Gel Process with Polyvinylpyrrolidone Additive. International Journal of Molecular Sciences. 2009; 10(9):4088-4101. https://doi.org/10.3390/ijms10094088
Chicago/Turabian StyleGarcía-Hernández, Margarita, Antonieta García-Murillo, Felipe de J. Carrillo-Romo, David Jaramillo-Vigueras, Geneviève Chadeyron, Elder De la Rosa, and Damien Boyer. 2009. "Eu-Doped BaTiO3 Powder and Film from Sol-Gel Process with Polyvinylpyrrolidone Additive" International Journal of Molecular Sciences 10, no. 9: 4088-4101. https://doi.org/10.3390/ijms10094088



