1. Introduction
From its first conception, envisioning technology beyond the microscopic level, below approximately 100 nm, nanotechnology has seen slow but steady development over the past five decades. Nanotechnological concepts and the fascinating possibilities that might arise when it would become possible to manipulate matter at the atomic scale were originally described in a lecture titled “There’s plenty of room at the bottom”, delivered at an American Physical Society meeting in 1959 by the physicist Richard Feynman, a Nobel Prize-laureate in 1965. The term “nanotechnology” was first defined by Norio Taniguchi in 1974 when he wrote that the term was derived from nanometer and defined “nanotechnology” as follows: “Nanotechnology mainly consists of the processing of, separation, consolidation, and deformation of materials by one atom or by one molecule” [
1]. The nanoscale—one billionth of a meter (10
−9 m)—marks the hazy boundary between the classical, Newtonian, and quantum mechanical worlds, and thus a variety of unique and deviant physical and chemical properties arise in nanoparticles compared with bulk materials.
At the outset it was envisioned that nanoscopically small devices could replace conventional industrial production methods and since such machines may convert matter at the atomic level to the desired end-product, a high substrate and product specificity, energy and conversion efficiency, and thus very little to no waste might be expected. These goals are still being pursued with significant scientific vigilance, but it is in a sense rather fitting that developments have been fastest in the biological and biomedical field, where substrate specificity, conversion efficiency, and product stereo-selectivity play crucial roles and are unparalleled endogenous biological traits. That such small devices are by no means the vision of mad scientists or science fiction writers, but already a reality is for instance exemplified by the engineering of nano-cars (
Figure 1): molecular chassis with fullerene wheels capable of motion and even directional control [
2].
In the biomedical field, nano-devices that might augment natural healing or defense processes, that could perform microsurgical tasks, actively and intelligently eradicate aberrant cells and tissues would constitute a revolution that surpasses all the milestones of the last centuries, such as the discovery in 1840 that ether gas can be used as a general anesthetic by Crawford Long and William Morton, Louis Pasteur’s unveiling of the artificial generation of weakened microorganisms in 1885—the principle was first established for the rabies vaccine—that paved the way for every other vaccine, or even the discovery of the first antibiotic, penicillin, by Sir Alexander Fleming in 1928. All these feats have saved the lives of millions of people.
Small nanoscopic medical devices that intelligently watch over the health of the individual—the stuff that Sci-Fi dreams are made of, such as the 1966 movie Fantastic Voyage, where Richard Fleischer envisioned to send surgeons in their miniaturized submarine into the body of an escaped Czech scientist to remove a blood clot in his brain, or more aptly considering the scope of this editorial, the “nanites” that autonomously regenerate tissue in injured Borg, used in Gene Roddenberry’s Star Trek series—are perhaps a long way of, but it is necessary to envision such goals for progress’ sake. On the other hand, the first “small step” may already have preceded “the giant leap” in current developments in nanoparticles that combine detectability, targeting, and therapeutic options in a single particle, i.e., “multifunctional” or “multimodal” probes. I personally prefer the latter term. Whether it is justified to already call this “thera(g)nostics” or not is a matter of debate, but fact is that such attributes have been demonstrated in a variety of nanoparticles by a number of laboratories.
Developments in this novel field of science at the boundary between physical, chemical, biological and medical science,
i.e., “Bionanotechnology”, have been relatively slow at the beginning, but momentum is taken up and current developments are progressing exponentially, as semi-empirically deducible from the increase in the yearly number of publications (
Figure 2). This normally is an indication that a new technology is maturing and this is certainly the case for a number of nanoparticles currently employed for research purposes. Evolutionary progress is complementary in the fields of nanotechnology and biotechnology, and there is a constant technological exchange and traffic between these. Nanoparticles are not only used to elucidate and study biological processes, but biomolecules, viruses, and cells are being used to fabricate and organize nano-materials that could not be produced
via synthetic techniques alone. The impact that nanostructured materials are already having on biological and biomedical research is profound. One of the most exciting applications are imageable nanoparticles, foremost fluorescent semiconducting quantum dots (Qdots).
2. Semiconductor Quantum Dots
Semiconducting nanocrystal technology was initially developed in the early 1980s in the labs of Louis Brus at Bell Laboratories [
4] and of Alexander Efros and Alexei I. Ekimov [
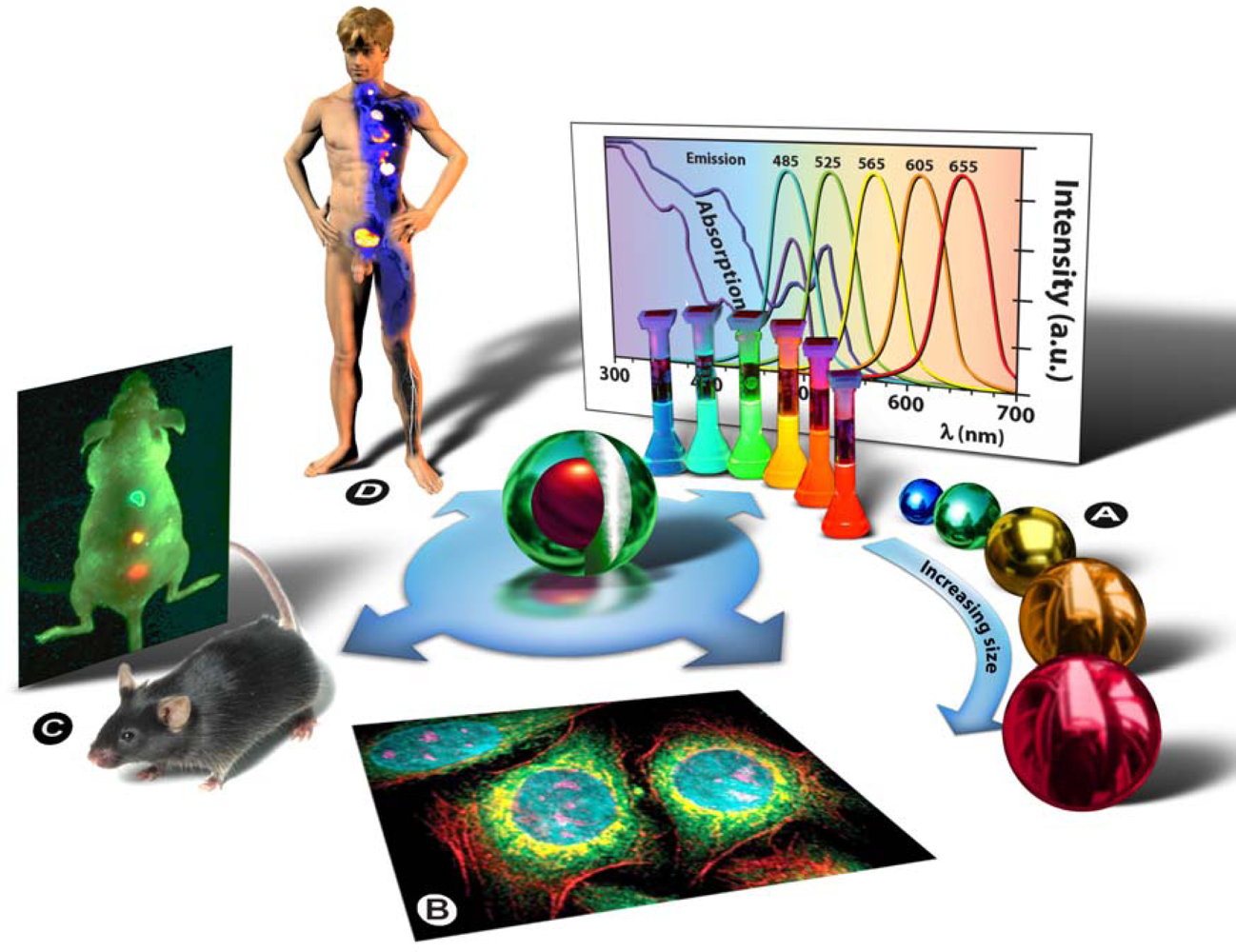
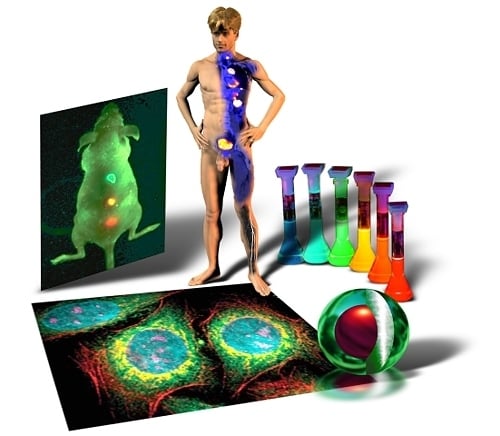
5] of the A.F. Ioffe Physical-Technical Institute in St. Petersburg. The term “quantum dot” was first contrived by Mark A. Reed in 1988 and denotes nanocrystalline semiconducting fluorophores, whose excitons are confined in all three spatial dimensions—quantum confinement: strict confinement of electrons and holes when the nanoparticle radius is below the exciton Bohr radius—and have typical diameters of 2–20 nm. Generally they are binary systems composed of a core of semiconducting material enclosed within a shell of another semiconductor (
Figure 3: Centre). Qdot fluorescence is caused by the bandgap between the valence and the conduction electron bands, and absorption of a photon higher in energy than the spectral bandgap of the core semiconductor results in electron excitation to the conduction band, generating an electron-hole pair (exciton). The long lifetime in the order of 10–40 ns increases the probability of absorption at shorter wavelengths, and produces a broad absorption spectrum (
Figure 3A). Since the physical size of the bandgap determines the photon’s emission wavelength, it is possible to control the fluorescence wavelength by the size of the nanoparticle (the bandgap energy is inversely proportional to the square of the size of the quantum dot). Simply put: the larger the Qdot, the redder its emission (
Figure 3A). Qdots are characterized by a number of unique physical properties, but since they are currently predominantly used as imaging agents it is particularly worth mentioning their optical properties: strong light absorbance, size-tunable emission, bright fluorescence/high quantum yield, narrow symmetric emission bands, high photostability and low photobleaching rates, and their broad absorption spectrum allows the simultaneous excitation of Qdots of all sizes by a single excitation light source in the UV to violet part of the spectrum (
Figure 3A).
Chemical adaptation of the nanoparticle surface not only renders the particle water-soluble, allows biocompatiblilization and functionalization, but also eliminates photobleaching by physically excluding interaction of the excited state particle with molecular oxygen and thus prevents formation of reactive oxygen species, such as singlet oxygen. By attaching particular targeting molecules, Qdots can be directed to sub-cellular structures (
Figure 3B) or even aberrant tissues in intact animals (
Figure 3C), and therefore can be used for bio-imaging purposes in cells [
6] and animals [
7,
8]. Next to bio-imaging applications, Qdots are currently being used in bio-analytical assays [
9] with a pure research focus. Ultimately, such strategies might be used in human patients to localize and treat diseased tissues and organs, as depicted in
figure 3D, or to replace conventional methods in clinical assays for diagnostic purposes. Drug delivery and therapeutic applications, such as photodynamic therapy [
10] and gene silencing [
11,
12], as well as applications that combine imaging capabilities with delivery of a therapeutic payload are presently being developed and tested in lab-animals. Clinical applications and trails in humans have not yet been performed pending resolution of remaining issues, such as tissue targeting, renal clearance, and toxicity issues.
3. Quo Vadis Quantum Dots
This special edition of the International Journal of Molecular Sciences (IJMS) brings together a number of articles dedicated to quantum dots and their application in life and biomedical sciences. Collectively, these articles illustrate recent advances in the field and highlight a promising and bright future for life- and biomedical sciences as well as the nanotechnological sciences that created them in the first place.
Maureen Walling and Jennifer Novak from Jason Shepard’s group, University at Albany (US) extensively review the application of quantum dots in live cell and
in vivo imaging [
13]. Their review focuses on imaging applications in single cell microscopy, tracking of individual cells, e.g., metastasis, and whole animal imaging and concomitant problems, as well as tackling the issue of targeting Qdots to particular (sub)cellular structures, cells, and tissues.
Jana Drbohlavova, Vojtech Adam, Rene Kizek, and Jaromir Hubalek, Brno University of Technology (CZ), center their review on the preparation and characterization of Qdots [
14]. The authors formulate several criteria for the safe use of Qdots in medicine and discuss short-comings in the current generation of Qdots.
Gerard Giraud
et al. from the University of Edinburgh (UK) in a collaborative effort between the Institute for Condensed Matter and Complex Systems, the Division of Pathway Medicine, the School of Chemistry, the Institute of Integrated Systems, and the National Physical Laboratory, describe the use of Qdot labeling combined with fluorescence lifetime imaging microscopy for the detection of DNA hybridization events on DNA microarrays [
15]. The authors show in this paper that exploitation of the relatively long lifetime of quantum dots with a FLIM imager that combines evanescent wave excitation with wide field detection and a quadrant anode mounted on an inverted microscope can be used to increase the contrast ratio of DNA microarrays by a factor of two.
In their paper, Raquel Ibáñez-Peral
et al. from the departments of Chemistry and Biomolecular Sciences, Earth Sciences, and Physics from Macquarie University and the School of Biotechnology and Biomolecular Sciences, University of New South Wales in Sydney (AU) investigate whether Qdots can be used as suitable multicolor optical labels of specific nucleotide probes for microbial identification using Flow Cytometry (FCM) [
17]. Since individual Qdots fluoresce and scatter below the resolution of the flow cytometer, the authors conjugated Qdots to paramagnetic beads (Dynabeads). They conclude that the minimum fluorophore-concentration required for detection of Qdots above the autofluorescent background was 100-fold less than for the commonly used fluorophore FITC, even under suboptimal excitation conditions. Nonetheless, their research also showed that Qdot binding to the beads markedly influences their optical properties and this fact alone suggests that the application of Qdots for FCM needs further study and development.
Takashi Jin and Yoshichika Yoshioka
et al. from the WPI Immunology Frontier Research Center, Osaka University (JP), in an excellent paper, describe preparation and characterization of glutathione (GSH)-coated near-infrared (NIR) Qdots for
in vivo fluorescence imaging [
18]. NIR probes are increasingly being used for deep tissue imaging, because they emit outside the autofluorescence window, and excitation beams in this region of the electromagnetic spectrum display better tissue penetrability and reduced photon scattering. Jin
et al. developed an easy synthesis route for GSH-coated NIR Qdots with a core-shell structure (CdSeTe/CdS) that can be used in cellular imaging, but also in intact animals, as demonstrated by lymph node imaging in a C57BL/6J mouse—the most widely used inbred strain refractory to many tumors, characterized by resistance to audiogenic seizures, relatively low bone density, development of age-related hearing loss, susceptibility to diet-induced obesity, type 2 diabetes, and atherosclerosis [
19].
To conclude, Ming-Shu Hsieh and Nion-Heng Shiao from Wen-Hsiung Chan’ lab, Department of Bioscience Technology and Center for Nanotechnology, Chung Yuan Christian University (TW), report on the influence of CdSe-core Qdots on mouse oocyte maturation, fertilization, and subsequent pre- and post-implantation fetal development [
20]. The authors show that exposure of mouse oocytes to CdSe-core Qdots during
in vitro maturation decreases cell number, induces apoptosis, and inhibits post-implantation development. Encapsulation with a ZnS shell effectively abrogates cytotoxic and teratogenic effects, demonstrating not only the potential toxicity in type I Qdots, but also the influence of the outer shielding.