Foam Properties and Detergent Abilities of the Saponins from Camellia oleifera
Abstract
:1. Introduction
2. Results and Discussion
2.1. Saponins Extract from C. oleifera
2.2. Foam Power of the Crude Saponins
2.3. Foam Stability of the Crude Saponins
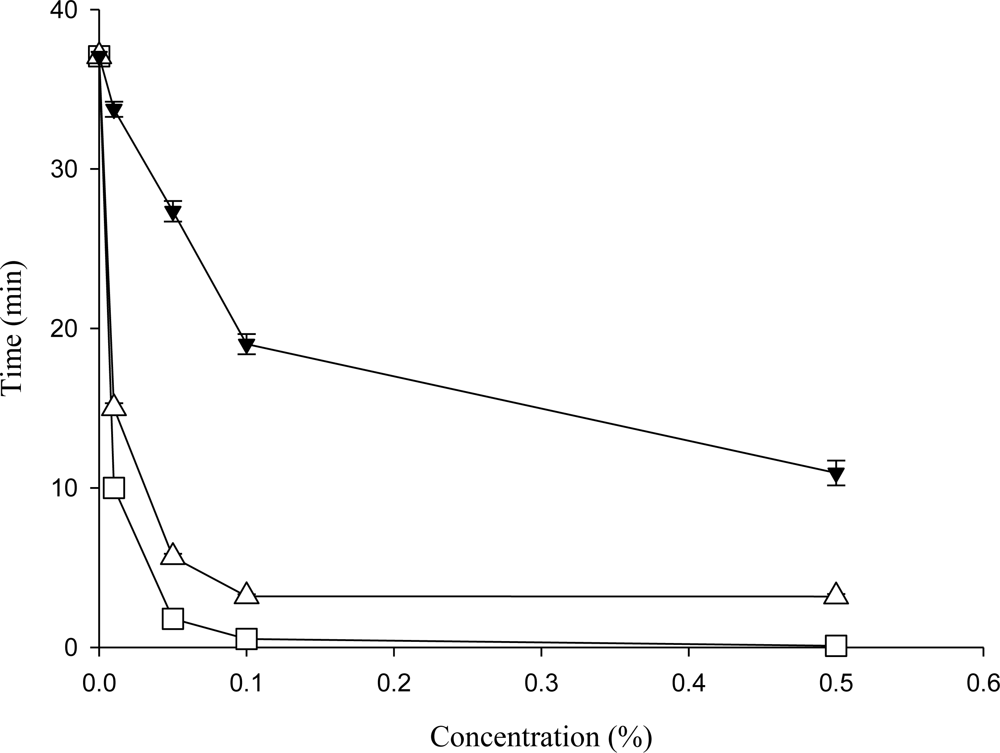
2.4. Wetting Ability of the Crude Saponins
2.5. Surface Tension of the Crude Saponins
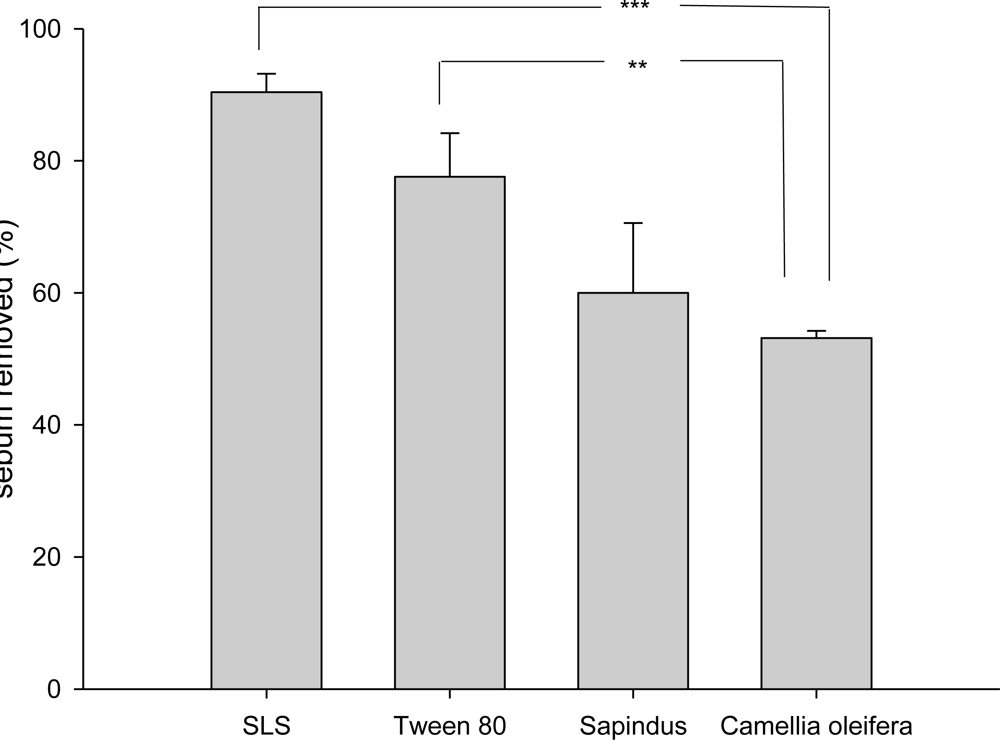
2.6. Detergent Ability of the Crude Saponins
3. Experimental Section
3.1. Chemicals
3.2. Determination of Total Saponins
3.3. Foaming Properties
3.4. Wetting Ability
3.5. Surface Tension
3.6. Detergent Ability
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Makkar, HPS; Siddhuraju, P; Becker, K. Methods in Molecular Biology: Plant Secondary Metabolites; Humana Press: Totowa, NJ, USA, 2007; pp. 93–100. [Google Scholar]
- Price, KR; Johnson, IT; Fenwick, GR. The chemistry and biological significance of saponins in foods and feeding stuffs. CRC Crit. Rev. Food Sci. Nutr 1987, 26, 127–135. [Google Scholar]
- Oakenfull, D. Saponins in food-A review. Food Chem 1981, 7, 19–40. [Google Scholar]
- Martin, RS; Briones, R. Industrial uses and sustainable supply of Quillaja saponaria (Rosaceae) saponins. Econ. Bot 1999, 53, 302–311. [Google Scholar]
- Tanaka, O; Tamura, Y; Masuda, H; Mizutani, K. Saponins Used in Food and Agriculture; Waller, GR, Yamasaki, K, Eds.; Plenum Press: New York, NY, USA, 1996; pp. 1–11. [Google Scholar]
- Huang, HC; Liao, SC; Chang, FR; Kuo, YH; Wu, YC. Molluscicidal saponins from Sapindus mukorossi, inhibitory agents of golden apple snails, Pomacea canaliculata. J. Agric. Food Chem 2003, 51, 4916–4919. [Google Scholar]
- Takagi, K; Park, EH; Kato, H. Anti-inflammatory activities of hederagenin and crude saponin isolated from Sapindus mukorossi Gaertn. Chem. Pharm. Bull 1980, 28, 1183–1188. [Google Scholar]
- Tamura, Y; Mizutani, K; Ikeda, T; Ohtani, K; Kasai, R; Yamasaki, K; Tanaka, O. Antimicrobial activities of saponins of pericarps of Sapindus mukurossi on dermatophytes. Nat. Med 2001, 55, 11–16. [Google Scholar]
- Sparg, SG; Light, ME; Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol 2004, 94, 219–243. [Google Scholar]
- Huang, Q; Shao, L; He, M; Chen, H; Liu, D; Luo, Y; Dai, Y. Inhibitory effects of sasanquasaponin on over-expression of ICAM-1 and on enhancement of capillary permeability induced by burns in rats. Burns 2005, 31, 637–642. [Google Scholar]
- Huang, Q; He, M; Chen, H; Shao, L; Liu, D; Luo, Y; Dai, Y. Protective effects of sasanquasaponin on injury of endothelial cells induced by anoxia and reoxygenation in vitro. Basic Clin. Pharmacol. Toxicol 2007, 101, 301–308. [Google Scholar]
- Chen, H; He, M; Huang, Q; Liu, D; Huang, M. Sasanquasaponin protects rat cardiomycytes against oxidatice stress induced by anoxia-reoxygenation injury. Eur. J. Pharmacol 2007, 575, 21–27. [Google Scholar]
- Tang, YA. The use of saponin to control predaceous fish in shrimp ponds. Prog. Fish Cult 1961, 23, 43–45. [Google Scholar]
- Chaicharoenpong, C; Petsom, A. Quantitative thin layer chromatographic analysis of the saponins in tea seed meal. Phytochem. Anal 2009, 20, 253–255. [Google Scholar]
- Mainkar, AR; Jolly, CI. Evaluation of commercial herbal shampoos. Int. J. Cosmet. Sci 2000, 22, 385–391. [Google Scholar]
- Xi, MM; Hai, CX; Tang, HF; Chen, MS; Fang, KQ; Xin, L. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother. Res 2008, 22, 228–237. [Google Scholar]
- Mousli, R; Tazerouti, A. Direct method of preparation of dodecanesulfonamide derivatives and some surface properties. J. Surf. Deterg 2007, 10, 279–285. [Google Scholar]
- Lunkenheimer, K; Malysa, K. Simple and generally applicable method of determination and evaluation of foam properties. J. Surf. Deterg 2003, 6, 69–74. [Google Scholar]
- Yang, CH; Huang, YC; Chen, YF; Zhang, MX. Foam properties, detergent abilities and long-term preservative efficacy of the saponins from Sapindus mukorossi. J. Food Drug Anal 2010, 18, 155–160. [Google Scholar]
- Hiai, S; Oura, H; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med 1976, 29, 116–122. [Google Scholar]
- Shiau, IL; Shih, TL; Wang, YN; Chen, HT; Lan, HF; Lin, HC; Yang, BY; Ko, CH; Murase, Y. Quantification for saponin from a soapberry in cleaning products by a chromatographic and two colorimetric assays. J. Fac. Agr. Kyushu. Univ 2009, 54, 215–221. [Google Scholar]
- Ross, J; Miles, GD. An apparatus for comparison of foaming properties of soaps and detergents. J. Am. Oil Chem. Soc 1941, 18, 99–102. [Google Scholar]
- Draves, CZ. Evaluation of wetting agents-official methods. Am. Dyestuff. Rep 1939, 28, 421–424. [Google Scholar]
- Badawi, AM; Mekawi, MA; Mohamed, AS; Mohamed, MZ; Khowdairy, MM. Surface and biological activity of some novel cationic surfactants. J. Surf. Deterg 2007, 10, 243–255. [Google Scholar]
- Thompson, D; Lemaster, C; Allen, R; Whittam, J. Evaluation of relative shampoo detergency. J. Soc. Cosmet. Chem 1985, 36, 271–286. [Google Scholar]


| Solution (0.5%) | Foam height (cm) | R5* | |
|---|---|---|---|
| 0 min | 5 min | ||
| SLS | 18.8 ± 0.21 | 17.6 ± 0.19 | 93.6% |
| Crude saponins extract | 4.57±0.10 | 3.93±0.05 | 86.0% |
| Tween 80 | 13.6 ± 0.15 | 13.1 ± 0.14 | 96.3% |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.-F.; Yang, C.-H.; Chang, M.-S.; Ciou, Y.-P.; Huang, Y.-C. Foam Properties and Detergent Abilities of the Saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417-4425. https://doi.org/10.3390/ijms11114417
Chen Y-F, Yang C-H, Chang M-S, Ciou Y-P, Huang Y-C. Foam Properties and Detergent Abilities of the Saponins from Camellia oleifera. International Journal of Molecular Sciences. 2010; 11(11):4417-4425. https://doi.org/10.3390/ijms11114417
Chicago/Turabian StyleChen, Yu-Fen, Chao-Hsun Yang, Ming-Shiang Chang, Yong-Ping Ciou, and Yu-Chun Huang. 2010. "Foam Properties and Detergent Abilities of the Saponins from Camellia oleifera" International Journal of Molecular Sciences 11, no. 11: 4417-4425. https://doi.org/10.3390/ijms11114417





