Lewis (y) Antigen Overexpression Increases the Expression of MMP-2 and MMP-9 and Invasion of Human Ovarian Cancer Cells
Abstract
:1. Introduction
2. Results
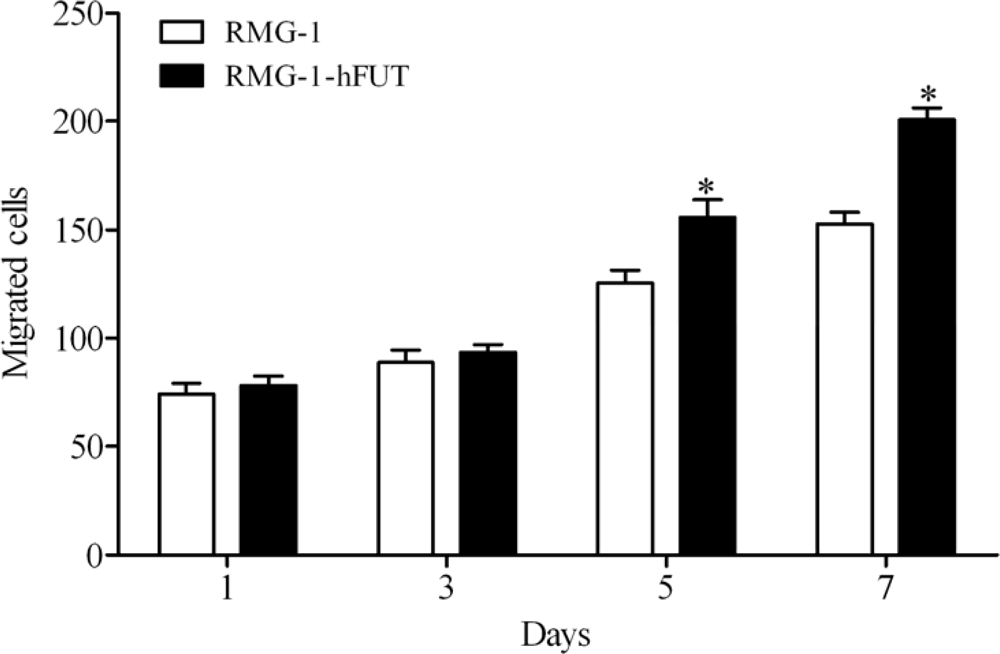
2.1. Overexpression of Lewis (y) Antigen Enhances Migration of RMG-1 Ovarian Cancer Cells
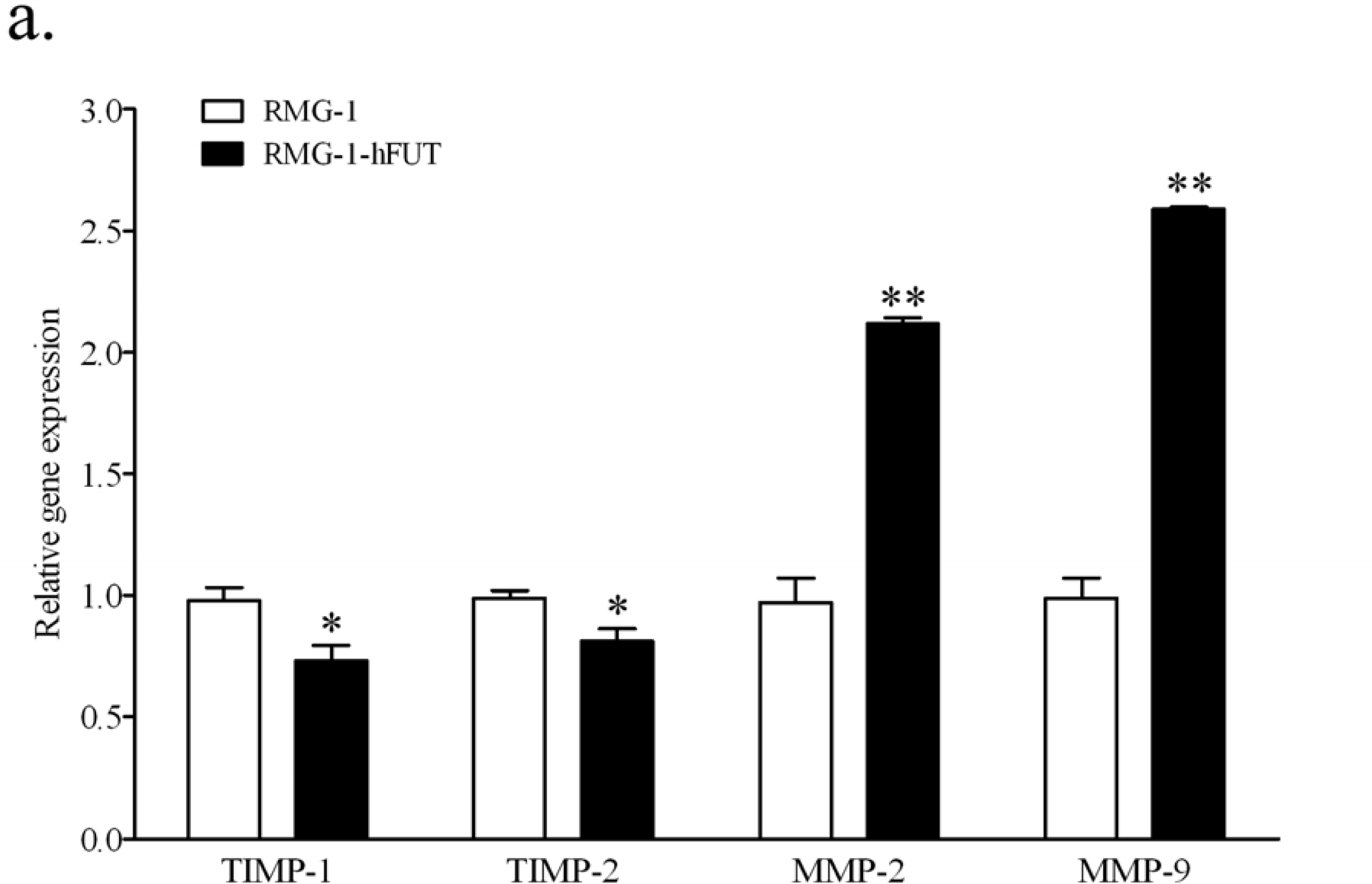
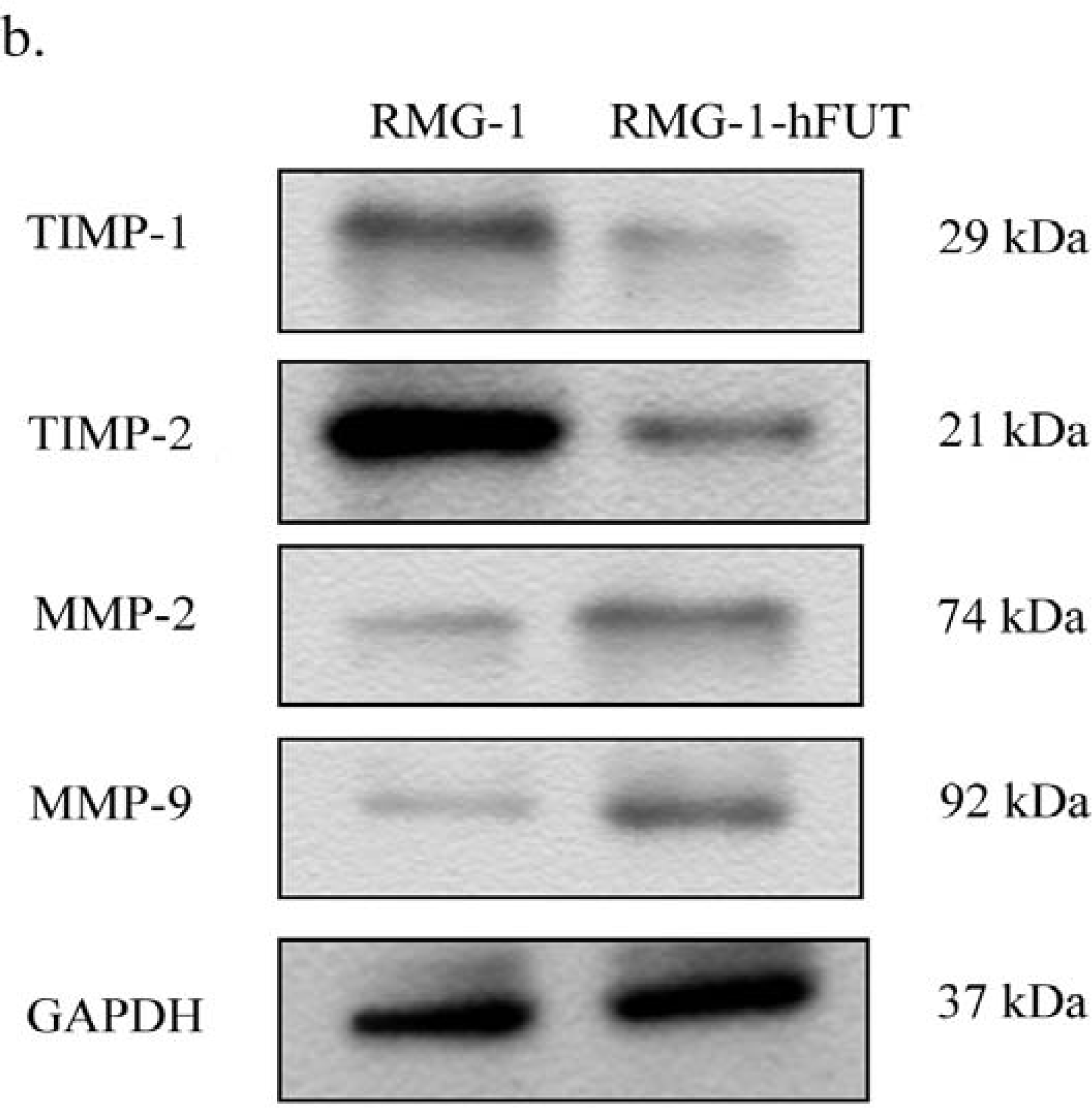
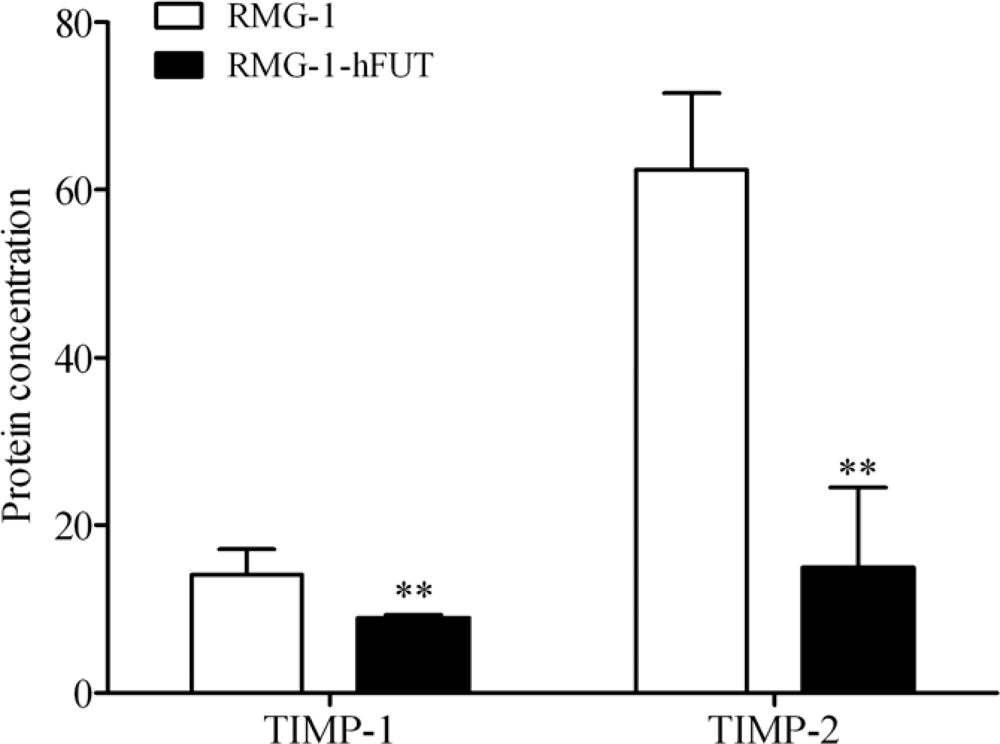
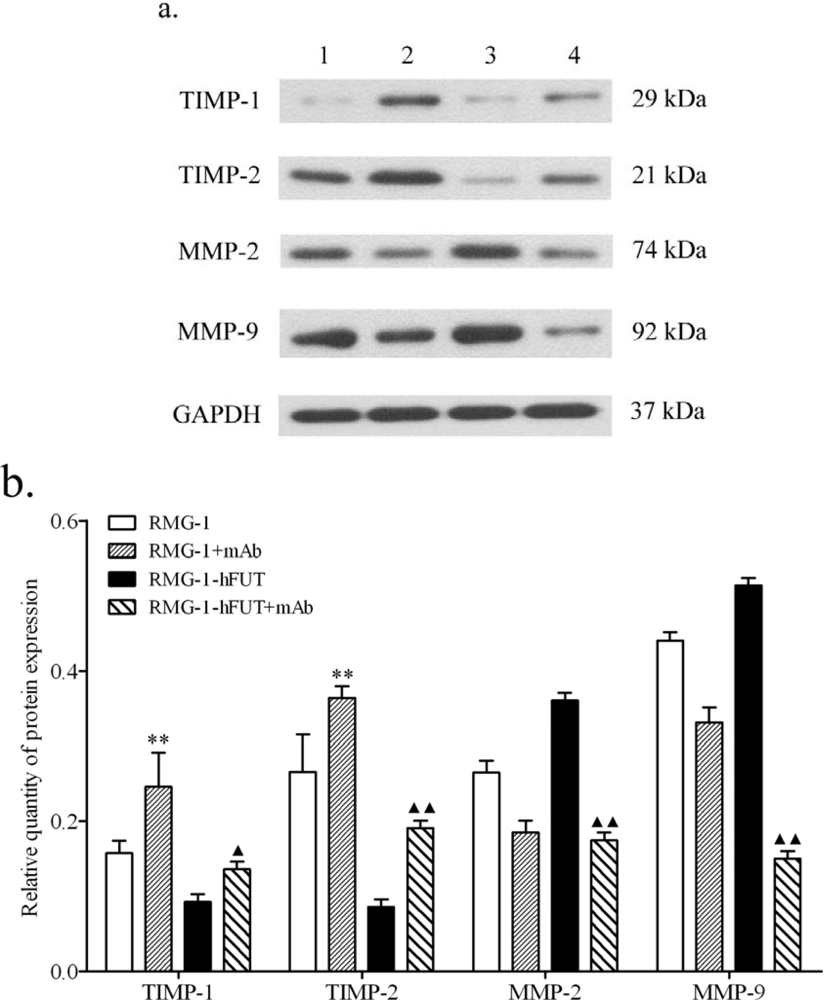
2.2. Down-Regulation of TIMPs and up-Regulation of MMPs by Lewis (y) Antigen
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. RNA Isolation and Quantitative Real-Time RT-PCR
4.3. Western Blot Analysis
4.4. Cell Migration Assay
4.5. Anti-Lewis (y) Antigen Antibody Blocking Test
4.6. ELISA
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
References
- Kitamura, K; Stockert, E; Garin-Chesa, P; Welt, S; Lloyd, KO; Armour, KL; Wallace, TP; Harris, WJ; Carr, FJ; Old, LJ. Specificity analysis of blood group Lewis-y (Le(y)) antibodies generatedagainst synthetic and natural Le(y) determinants. Proc. Natl. Acad. Sci., USA 1994, 91, 12957–12961. [Google Scholar]
- Hokke, CH; Neeleman, AP; Koeleman, CA; van den Eijnden, DH. Identification of an alpha3-fucosyltransferase and a novel alpha2-fucosyltransferase activity in cercariae of the schistosome Trichobilharzia ocellata: Biosynthesis of the Fucalpha1-->2Fucalpha1-->3[Gal(NAc)beta1-->4]GlcNAc sequence. Glycobiology 1998, 8, 393–406. [Google Scholar]
- Dettke, M; Palfi, G; Loibner, H. Activation-dependent expression of the blood group-related lewis Y antigen on peripheral blood granulocytes. J. Leukoc. Biol 2000, 68, 511–514. [Google Scholar]
- Hellstrom, I; Garrigues, HJ; Garrigues, U; Hellstrom, KE. Highly tumor-reactive, internalizing, mouse monoclonal antibodies to Le(y)-related cell surface antigens. Cancer Res 1990, 50, 2183–2190. [Google Scholar]
- Madjd, Z; Parsons, T; Watson, NF; Spendlove, I; Ellis, I; Durrant, LG. High expression of Lewis y/b antigens is associated with decreased survival in lymph node negative breast carcinomas. Breast Cancer Res 2005, 7, R780–R787. [Google Scholar]
- Zhu, LC; Lin, B; Hao, YY; Li, FF; Diao, B; Zhang, SL. Impact of alpha1,2-fucosyl transferase gene transfection on cancer-related gene expression profile of human ovarian cancer cell line RMG-1. Ai Zheng 2008, 27, 934–941. [Google Scholar]
- Vernon, AE; LaBonne, C. Tumor metastasis: A new twist on epithelial-mesenchymal transitions. Curr. Biol 2004, 14, R719–R721. [Google Scholar]
- Vazquez-Ortiz, G; Pina-Sanchez, P; Vazquez, K; Duenas, A; Taja, L; Mendoza, P; Garcia, JA; Salcedo, M. Overexpression of cathepsin F, matrix metalloproteinases 11 and 12 in cervical cancer. BMC Cancer 2005, 5. doi:10.1186/1471-2407-5-68. [Google Scholar]
- Visse, R; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res 2003, 92, 827–839. [Google Scholar]
- Talvensaari-Mattila, A; Paakko, P; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br. J. Cancer 2003, 89, 1270–1275. [Google Scholar]
- Pellikainen, JM; Ropponen, KM; Kataja, VV; Kellokoski, JK; Eskelinen, MJ; Kosma, VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin. Cancer Res 2004, 10, 7621–7628. [Google Scholar]
- Kim, MH; Kitson, RP; Albertsson, P; Nannmark, U; Basse, PH; Kuppen, PJ; Hokland, ME; Goldfarb, RH. Secreted and membrane-associated matrix metalloproteinases of IL-2-activated NK cells and their inhibitors. J. Immunol 2000, 164, 5883–5889. [Google Scholar]
- Lu, KV; Jong, KA; Rajasekaran, AK; Cloughesy, TF; Mischel, PS. Upregulation of tissue inhibitor of metalloproteinases (TIMP)-2 promotes matrix metalloproteinase (MMP)-2 activation and cell invasion in a human glioblastoma cell line. Lab. Invest 2004, 84, 8–20. [Google Scholar]
- van den Steen, PE; Dubois, B; Nelissen, I; Rudd, PM; Dwek, RA; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol 2002, 37, 375–536. [Google Scholar]
- Chang, C; Werb, Z. The many faces of metalloproteases: Cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol 2001, 11, S37–43. [Google Scholar]
- Yan, LM; Lin, B; Zhu, LC; Hao, YY; Qi, Y; Wang, CZ; Gao, S; Liu, SC; Zhang, SL; Iwamori, M. Enhancement of the adhesive and spreading potentials of ovarian carcinoma RMG-1 cells due to increased expression of integrin alpha5beta1 with the Lewis Y-structure on transfection of the alpha1,2-fucosyltransferase gene. Biochimie 2010, 92, 852–857. [Google Scholar]
- Gao, S; Liu, Q; Wang, X; Lin, B; Zhang, S. Effects of Lewis Y antigen on the gene expression of multiple drug resistance-associated proteins in human ovarian cancer RMG-I-H cells. Med. Oncol 2010, 27, 960–967. [Google Scholar]
- Liu, J; Lin, B; Hao, Y; Qi, Y; Zhu, L; Li, F; Liu, D; Cong, J; Zhang, S; Iwamori, M. Lewis y antigen promotes the proliferation of ovarian carcinoma-derived RMG-I cells through the PI3K/Akt signaling pathway. J. Exp. Clin. Cancer Res 2009, 28, 154. [Google Scholar]
- Liu, JJ; Lin, B; Hao, YY; Li, FF; Liu, DW; Qi, Y; Zhu, LC; Zhang, SL; Iwamori, M. Lewis(y) antigen stimulates the growth of ovarian cancer cells via regulation of the epidermal growth factor receptor pathway. Oncol. Rep 2010, 23, 833–841. [Google Scholar]
- Redondo-Munoz, J; Ugarte-Berzal, E; Terol, MJ; van den Steen, PE; del Cerro, MH; Roderfeld, M; Roeb, E; Opdenakker, G; García-Marco, JA; García-Pardo, A. Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia b cell survival through its hemopexin domain. Cancer Cell 2010, 17, 160–172. [Google Scholar]
- O-Charoenrat, P; Rhys-Evans, P; Modjtahedi, H; Court, W; Box, G; Eccles, S. Overexpression of epidermal growth factor receptor in human head and neck squamous carcinoma cell lines correlates with matrix metalloproteinase-9 expression and in vitro invasion. Int. J. Cancer 2000, 86, 307–317. [Google Scholar]
- Choi, YA; Lim, HK; Kim, JR; Lee, CH; Kim, YJ; Kang, SS; Baek, SH. Group IB secretory phospholipase A2 promotes matrix metalloproteinase-2-mediated cell migration via the phosphatidylinositol 3-kinase and Akt pathway. J. Biol. Chem 2004, 279, 36579–36585. [Google Scholar]
| Gene symbol | Sequences | Product size (bp) |
|---|---|---|
| TIMP-1 | F: 5’-GTTGTTGCTGTGGCTGATAG-3’ | 266 |
| R: 5’-TGTGGGACCTGTGGAAGTA-3’ | ||
| TIMP-2 | F: 5’-CGCTCAAATACCTTCACAA-3’ | 217 |
| R: 5’-CGGCAGCAAGTCCAATA-3’ | ||
| GAPDH | F: 5’-AAGGCTGTGGGCAAGG-3’ | 238 |
| R: 5’-TGGAGGAGTGGGTGTCG-3’ | ||
| MMP-2 | F: 5’-TTGACGGTAAGGACGGACTC-3’ | 153 |
| R: 5’-ACTTGCAGTACTCCCCATCG-3’ | ||
| MMP-9 | F: 5’-TTGACAGCGACAAGAAGTGG-3’ | 179 |
| R: 5’-GCCATTCACGTCGTCCTTAT-3’ |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yan, L.; Lin, B.; Gao, L.; Gao, S.; Liu, C.; Wang, C.; Wang, Y.; Zhang, S.; Iwamori, M. Lewis (y) Antigen Overexpression Increases the Expression of MMP-2 and MMP-9 and Invasion of Human Ovarian Cancer Cells. Int. J. Mol. Sci. 2010, 11, 4441-4451. https://doi.org/10.3390/ijms11114441
Yan L, Lin B, Gao L, Gao S, Liu C, Wang C, Wang Y, Zhang S, Iwamori M. Lewis (y) Antigen Overexpression Increases the Expression of MMP-2 and MMP-9 and Invasion of Human Ovarian Cancer Cells. International Journal of Molecular Sciences. 2010; 11(11):4441-4451. https://doi.org/10.3390/ijms11114441
Chicago/Turabian StyleYan, Limei, Bei Lin, Lili Gao, Song Gao, Chuan Liu, Changzhi Wang, Yifei Wang, Shulan Zhang, and Masao Iwamori. 2010. "Lewis (y) Antigen Overexpression Increases the Expression of MMP-2 and MMP-9 and Invasion of Human Ovarian Cancer Cells" International Journal of Molecular Sciences 11, no. 11: 4441-4451. https://doi.org/10.3390/ijms11114441




