Comparison of the Phenolic Content and Antioxidant Activities of Apocynum venetum L. (Luo-Bu-Ma) and Two of Its Alternative Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Total Flavonoid Content
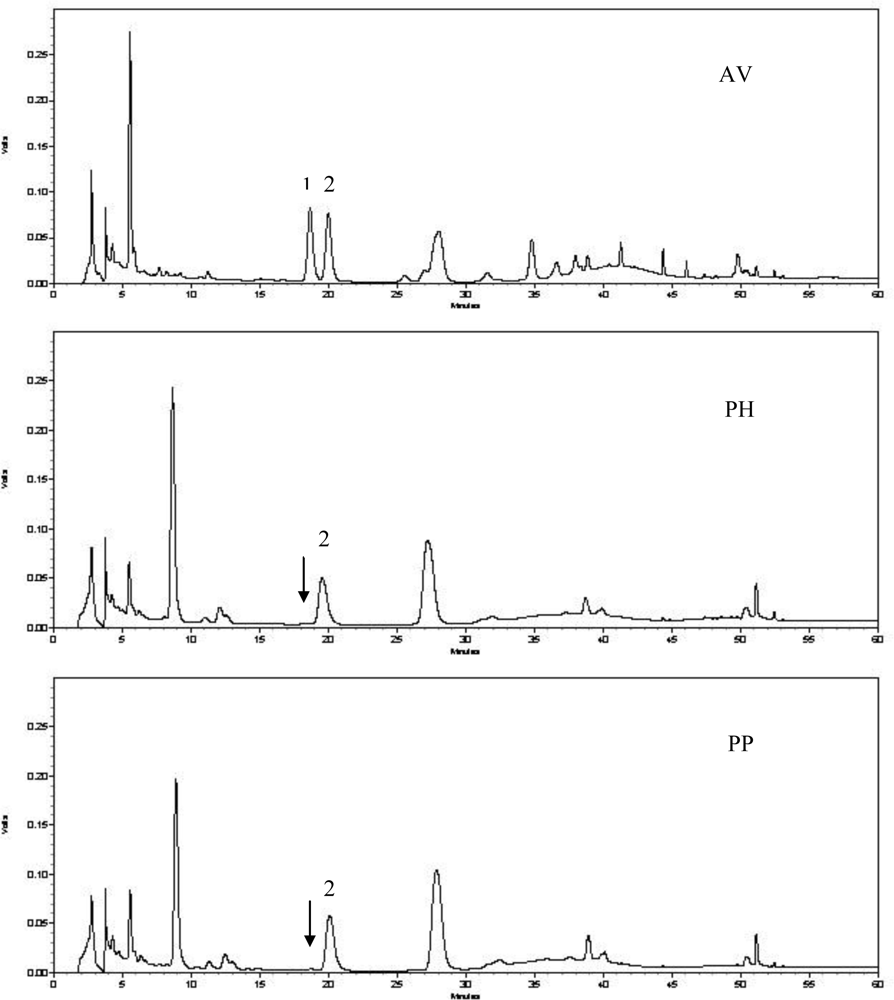
2.2. Quantitative Analysis of Hyperoside and Isoquercitrin
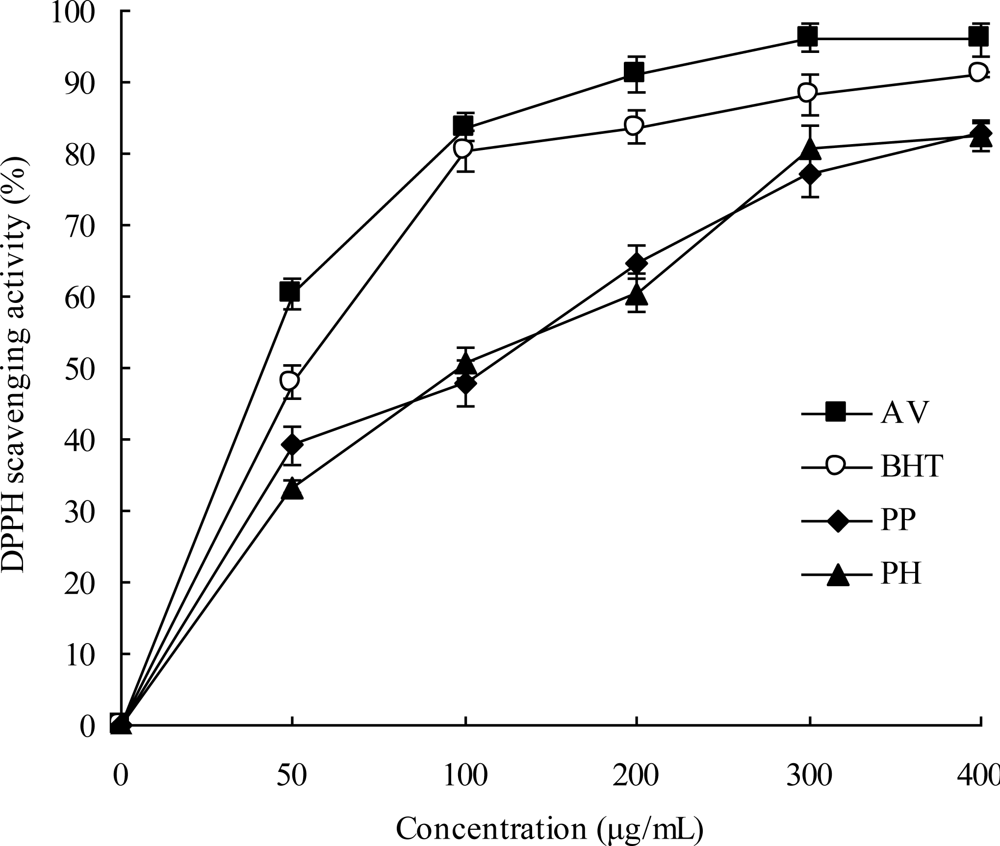
2.3. DPPH Radical Scavenging Activities
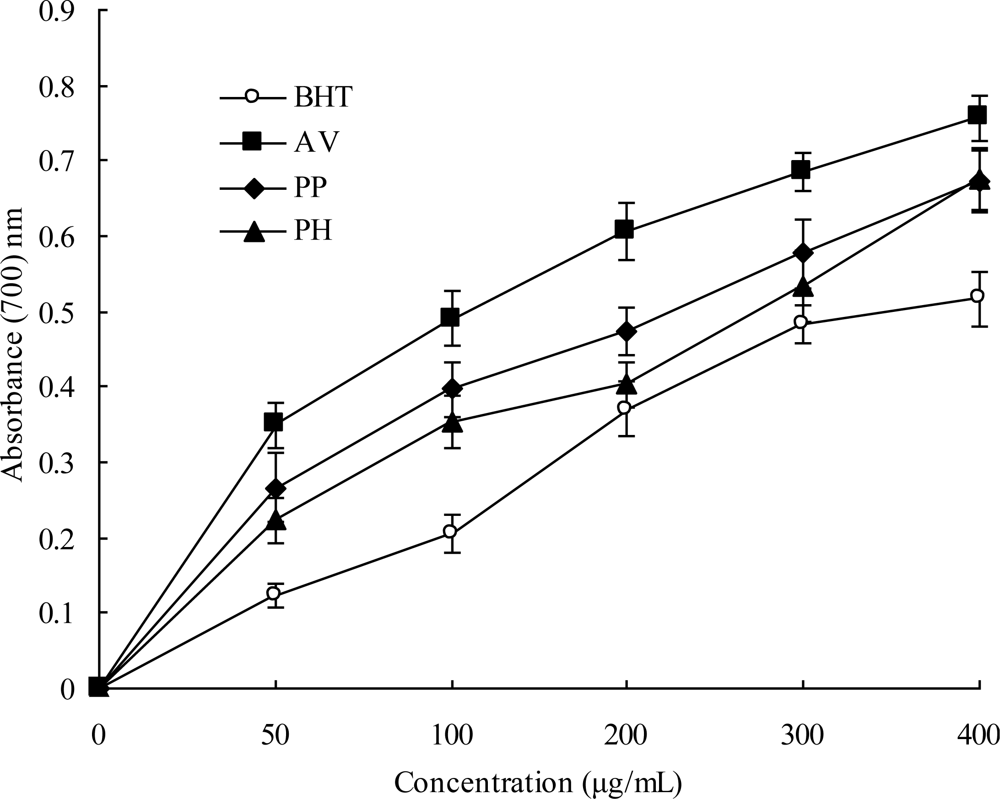
2.4. Reducing Power
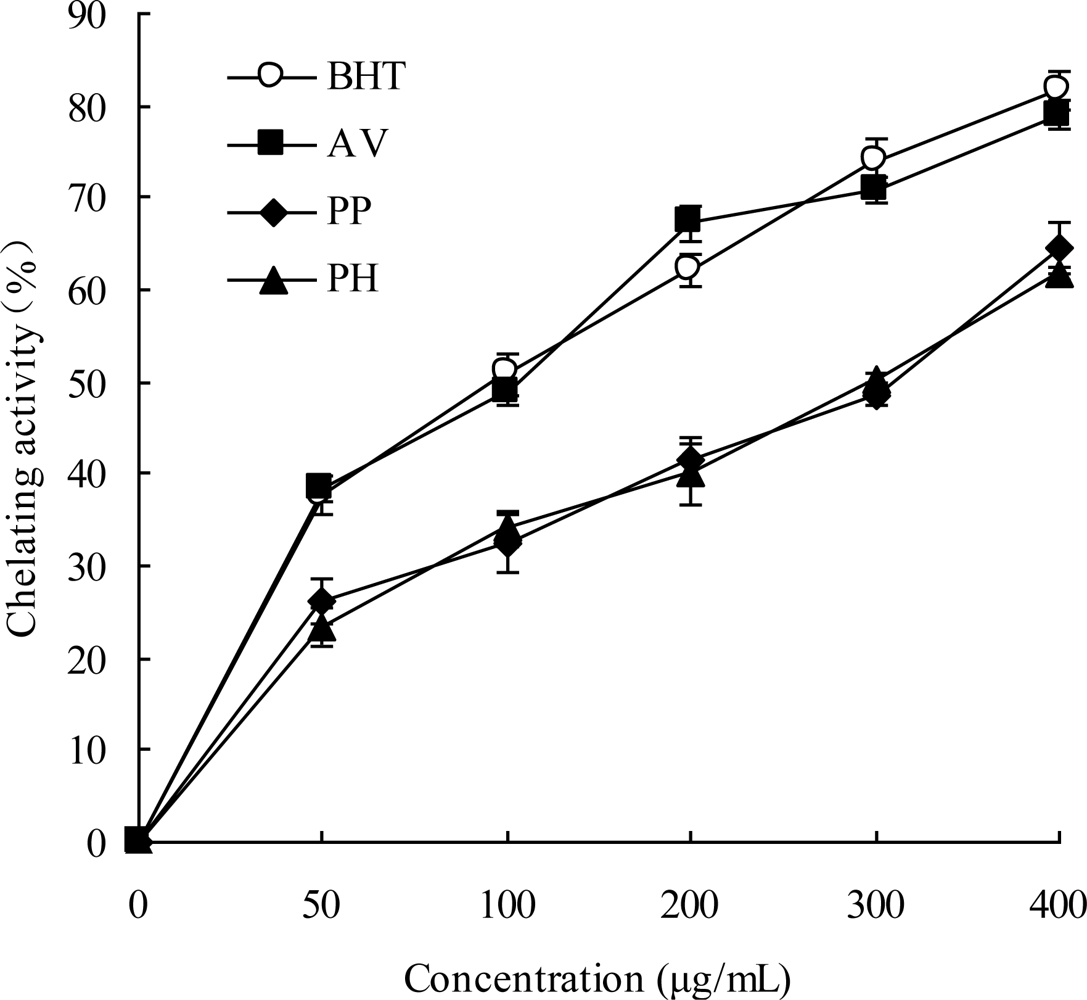
2.5. Chelating Ability of Ferrous Ions
3. Experimental Section
3.1. Plant Materials
3.2. Standards and Reagents
3.3. Equipment and Apparatus
3.4. Preparation of Methanolic Extracts
3.5. Determination of Total Phenolics Content
3.6. Determination of Total Flavonoids Content
3.7. HPLC Determination of Hyperoside and Isoquercitrin
3.8. DPPH Radical Scavenging Activity
3.9. Reducing Power
3.10. Chelating Ability of Ferrous Ions
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Halliwell, B. Free radicals, proteins and DNA: Oxidative damage versus redox regulation. Biochem. Soc. Trans 1996, 24, 1023–1027. [Google Scholar]
- Dalle, DI; Scaloni, A; Giustarini, D; Cavarra, E; Tell, G; Lungarella, G; Colombo, R; Rossi, R; Milzani, A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: The contribution of redox proteomics. Mass Spectrom. Rev 2005, 24, 55–99. [Google Scholar]
- Muralikrishna, AR; Hatcher, JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic. Biol. Med 2006, 40, 376–387. [Google Scholar]
- Trachtenberg, BH; Hare, JM. Biomarkers of oxidative stress in heart failure. Heart Fail. Clin 2009, 5, 561–577. [Google Scholar]
- Furukawa, S; Fujita, T; Shimabukuro, M; Iwaki, M; Yamada, Y; Nakajima, Y; Nakayama, O; Makishima, M; Matsuda, M; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest 2004, 114, 1752–1761. [Google Scholar]
- Cesaratto, L; Vascotto, C; Calligaris, S; Tell, G. The importance of redox state in liver damage. Ann. Hepatol 2004, 3, 86–92. [Google Scholar]
- Sadani, GR; Nadkarni, GD. Role of tissue antioxidant defence in thyroid cancers. Cancer Lett 1996, 109, 231–235. [Google Scholar]
- Lindsay, DG. Diet and ageing: the possible relation to reactive oxygen species. J. Nutr. Health Aging 1999, 3, 84–91. [Google Scholar]
- Shukla, S; Mehta, A; John, J; Singh, S; Mehta, P; Vyas, SP. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem. Toxicol 2009, 47, 1848–1851. [Google Scholar]
- Safer, AM; Al-Nughamish, AJ. Hepatotoxicity induced by the anti-oxidant food additive, butylated hydroxytoluene (BHT), in rats: an electron microscopical study. Histol. Histopathol 1999, 14, 391–406. [Google Scholar]
- Branen, AL. Toxicology and biochemistry of butylated hydroxyl anisole and butylated hydroxytoluene. J. Am. Oil Chem. Soc 1975, 52, 59–63. [Google Scholar]
- Liao, H; Banbury, LK; Leach, DN. Antioxidant activity of 45 Chinese herbs and the relationship with their TCM characteristics. Evidence-based Compl. Alt. Med 2008, 5, 429–434. [Google Scholar]
- Wang, KJ; Zhang, YJ; Yang, CR. Antioxidant phenolic constituents from Fagopyrum dibotrys. J. Ethnopharmacol 2005, 99, 259–264. [Google Scholar]
- Complication of Luobuma Utilization Edited Group, Total Utilization of Luobuma; Science Press: Beijing, China, 1978; pp. 57–92.
- Butterweck, V; Nishibe, S; Sasaki, T; Uchida, M. Antidepressant effects of Apocynum venetum leaves in a forced swimming test. Biol. Pharm. Bull 2001, 24, 848–851. [Google Scholar]
- Grundmann, O; Nakajima, J; Seo, S; Butterweck, V. Antianxiety effects of Apocynum venetum L. in the elevated plus maze test. J. Ethnopharmacol 2007, 110, 406–441. [Google Scholar]
- Yokozawa, T; Kashiwada, Y; Hattori, M; Chung, HY. Study on the components of luobuma with peroxynitrite-scavenging activity. Biol. Pharm. Bull 2002, 25, 748–752. [Google Scholar]
- Shirai, M; Kawai, Y; Yamanishi, R; Terao, J. Approach to novel functional foods for stress control 5. Antioxidant activity profiles of antidepressant herbs and their active components. J Med Invest 2005, 52(Suppl.), 249–251. [Google Scholar]
- Zhou, CL; Sun, L; Bi, KS. RP-HPLC analysis of hyperoside and isoquercitrin in Apocynum venetum L. Chin. J. Pharm. Anal 2009, 29, 1001–1003. [Google Scholar]
- Piao, XL; Mi, XY; Tian, YZ; Wu, Q; Piao, HS; Zeng, Z; Wang, D; Piao, X. Rapid identification and characterization of antioxidants from Ligularia fischeri. Arch. Pharm. Res 2009, 32, 1689–1694. [Google Scholar]
- Shibano, M; Kakutani, K; Taniguchi, M; Yasuda, M; Baba, K. Antioxidant constituents in the dayflower (Commelina communis L.) and their alpha-glucosidase-inhibitory activity. J. Nat. Med 2008, 62, 349–353. [Google Scholar]
- Wang, J; Sun, B; Cao, Y; Tian, Y; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem 2008, 106, 804–810. [Google Scholar]
- Slinkard, K; Singleton, VL. Total phenol analyses: Automation and comparison with manual methods. Am. J. Enol. Vitic 1977, 28, 49–55. [Google Scholar]
- Wang, K; Pan, YM; Wang, HS; Zhang, Y; Lei, Q; Zhu, ZR; Li, HY; Liang, M. Antioxidant activities of Liquidambar formosana Hance leaf extracts. Med. Chem. Res 2010, 19, 166–176. [Google Scholar]
- Brand, WW; Cuvelier, ME; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol 1995, 28, 25–30. [Google Scholar]
- Zhu, QY; Hackman, RM; Ensunsa, JL; Holt, RR; Keen, CL. Antioxidative activities of oolong tea. J. Agric. Food Chem 2002, 50, 6229–6934. [Google Scholar]
- Dinis, TCP; Madeira, VMC; Almeida, LM. Action of phenolic derivates (acetoaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys 1994, 315, 161–169. [Google Scholar]
- Cazarolli, LH; Zanatta, L; Alberton, EH; Figueiredo, MS; Folador, P; Damazio, RG; Pizzolatti, MG; Silva, FR. Flavonoids: prospective drug candidates. Mini. Rev. Med. Chem 2008, 8, 1429–1440. [Google Scholar]
- Hoki, S; Kimura, T; Nagasawa, M; Kozaki, K; Terashima, N. Antihypertensive effects of the principal flavonoids of YANLONG tea in spontaneously hypertensive rats. Nat. Med 2004, 58, 113–116. [Google Scholar]
- Sancin, P. The phenolic compounds of underground parts of Apocynum venetum. Planta Med 1971, 20, 153–155. [Google Scholar]
- Xiong, Q; Fan, W; Tezuka, Y; Adnyana, IK; Stampoulis, P; Hattori, M; Namba, T; Kadota, S. Hepatoprotective effect of Apocynum venetum and its active constituents. Planta Med 2000, 66, 127–133. [Google Scholar]
- Kamata, K; Seo, S; Nakajima, J. Constituents from leaves of Apocynum venetum L. J. Nat. Med 2008, 62, 160–163. [Google Scholar]
- Davis, JM; Murphy, EA; Carmichael, MD. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sports Med. Rep 2009, 8, 206–213. [Google Scholar]
- Gomes, A; Fernandes, E; Lima, JL; Mira, L; Corvo, ML. Molecular mechanisms of anti-inflammatory activity mediated by flavonoids. Curr. Med. Chem 2008, 15, 1586–1605. [Google Scholar]
- Liu, WF; Mu, SX; Liu, XR; Feng, C. Determination of rutin and quercetin in Folium apocyni Veneti by RP-HPLC. Chin. Arch. Trad. Chin. Med 2009, 27, 2677–2678. [Google Scholar]
- Surveswaran, S; Cai, YZ; Corke, H; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem 2007, 102, 938–953. [Google Scholar]
- Costa, RM; Magalhães, AS; Pereira, JA; Andrade, PB; Valentão, P; Carvalho, M; Silva, BM. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: a comparative study with green tea (Camellia sinensis). Food Chem. Toxicol 2009, 47, 860–865. [Google Scholar]
- Irie, K; Sato, T; Tanaka, I; Nakajima, J; Kawaguchi, M; Himi, T. Cardiotonic effect of Apocynum venetum L. extracts on isolated guinea pig atrium. J. Nat. Med 2009, 63, 111–116. [Google Scholar]
- Hsu, CL; Chen, W; Weng, YM; Tseng, CY. Chemical composition, physical properties and antioxidant activities of yam flours as affected by different drying methods. Food Chem 2003, 83, 85–92. [Google Scholar]
- Odabasoglu, F; Aslan, A; Cakir, A; Suleyman, H; Karagoz, Y; Bayir, Y; Halici, M. Antioxidant activity, reducing power and total phenolic content of some lichen species. Fitoterapia 2005, 76, 216–219. [Google Scholar]
- Duh, PD; Du, PC; Yen, GC. Action of methanolic extract of mung bean hulls as inhibitors of lipid peroxidation and non-lipid oxidative damage. Food Chem. Toxicol 1999, 37, 1055–1061. [Google Scholar]
- You, Y; Duan, X; Wei, X; Su, X; Zhao, M; Sun, J; Ruenroengklin, N; Jiang, Y. Identification of major phenolic compounds of Chinese water chestnut and their antioxidant activity. Molecules 2007, 12, 842–852. [Google Scholar]
- Mehmet Öztürk, M; Aydoğmuş-Öztürk, F; Duru, ME; Topçud, G. Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): an edible medicinal plant. Food Chem 2007, 103, 623–630. [Google Scholar]
- Kaur, G; Alam, MS; Jabbar, Z; Javed, K; Athar, M. Evaluation of antioxidant activity of Cassia siamea flowers. J. Ethnopharmacol 2006, 108, 340–348. [Google Scholar]
- Meghashri, S; Kumar, HV; Gopal, S. Antioxidant properties of a novel flavonoid from leaves of Leucas aspera. Food Chem 2010, 122, 105–110. [Google Scholar]
- Hudson, BJF; Lewis, JI. Polyhydroxy flavonoid antioxidants for edible oils, structure-activity relationships. Food Chem 1983, 10, 47–55. [Google Scholar]
| Plant | Total phenolics content (mg GAE/g dry extract) | Total flavonoids content (mg RE/g dry extract) |
|---|---|---|
| AV | 92.71 ± 2.16a | 31.09 ± 1.73a |
| PP | 53.58 ± 0.81b | 17.02 ± 1.35b |
| PH | 50.61 ± 1.19b | 18.16 ± 1.04b |
| Plant | Hyperoside (mg/g dry extract) | Isoquercitrin (mg/g dry extract) |
|---|---|---|
| AV | 11.61 ± 1.02 | 13.62 ± 1.24a |
| PP | ND | 11.89 ± 1.13b |
| PH | ND | 12.10 ± 0.81b |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liang, T.; Yue, W.; Li, Q. Comparison of the Phenolic Content and Antioxidant Activities of Apocynum venetum L. (Luo-Bu-Ma) and Two of Its Alternative Species. Int. J. Mol. Sci. 2010, 11, 4452-4464. https://doi.org/10.3390/ijms11114452
Liang T, Yue W, Li Q. Comparison of the Phenolic Content and Antioxidant Activities of Apocynum venetum L. (Luo-Bu-Ma) and Two of Its Alternative Species. International Journal of Molecular Sciences. 2010; 11(11):4452-4464. https://doi.org/10.3390/ijms11114452
Chicago/Turabian StyleLiang, Taigang, Wenyan Yue, and Qingshan Li. 2010. "Comparison of the Phenolic Content and Antioxidant Activities of Apocynum venetum L. (Luo-Bu-Ma) and Two of Its Alternative Species" International Journal of Molecular Sciences 11, no. 11: 4452-4464. https://doi.org/10.3390/ijms11114452




