Insight into the Strong Antioxidant Activity of Deinoxanthin, a Unique Carotenoid in Deinococcus Radiodurans
Abstract
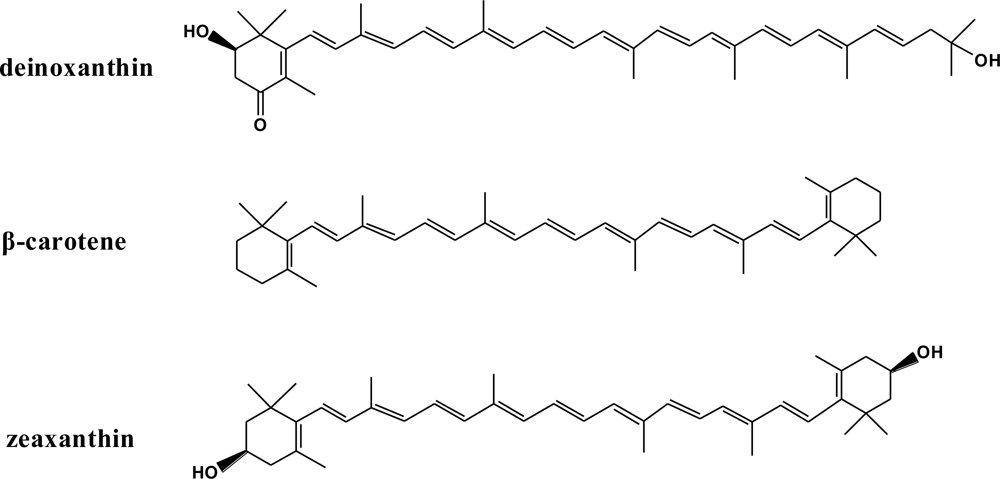
:1. Introduction
2. Calculation Methods
3. Results and Discussion
Acknowledgments
References
- Daly, MJ; Minton, KW. Interchromosomal recombination in the extremely radioresistant bacterium Deinococcus radiodurans. J. Bacteriol 1995, 177, 5495–5505. [Google Scholar]
- Cox, MM; Battista, JR. Deinococcus radiodurans—the consummate survivor. Nat. Rev. Microbiol 2005, 3, 882–892. [Google Scholar]
- Slade, D; Lindner, AB; Paul, G; Radman, M. Recombination and replication in DNA repair of heavily irradiated Deinococcus radiodurans. Cell 2009, 136, 1044–1055. [Google Scholar]
- Ghosal, D; Omelchenko, MV; Gaidamakova, EK; Matrosova, VY; Vasilenko, A; Venkateswaran, A; Zhai, M; Kostandarithes, HM; Brim, H; Makarova, KS; Wackett, LP; Fredrickson, JK; Daly, MJ. How radiation kills cells: survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress. FEMS Microbiol. Rev 2005, 29, 361–375. [Google Scholar]
- Markillie, LM; Varnum, SM; Hradecky, P; Wong, K. Targeted mutagenesis by duplication insertion in the radioresistant bacterium Deinococcus radiodurans: radiation sensitivities of catalase (katA) and superoxide dismutase (sodA) mutants. J. Bacteriol 1999, 181, 666–669. [Google Scholar]
- Paiva, SA; Russell, RM. Beta-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr 1999, 18, 426–433. [Google Scholar]
- Lemee, L; Peuchant, E; Clerc, M; Brunner, M; Pfander, H. Deinoxanthin: A new carotenoid isolated from Deinococcus radiodurans. Tetrahedron 1997, 53, 919–926. [Google Scholar]
- Saito, T; Ohyama, Y; Ide, H; Ohta, S; Yamamoto, O. A carotenoid pigment of the radioresistant bacterium Deinococcus radiodurans. Microbios 1998, 95, 79–90. [Google Scholar]
- Tian, B; Xu, Z; Sun, Z; Lin, J; Hua, Y. Evaluation of the antioxidant effects of carotenoids from Deinococcus radiodurans through targeted mutagenesis, chemiluminescence, and DNA damage analyses. Biochim. Biophys. Acta 2007, 1770, 902–911. [Google Scholar]
- Hohenberg, P; Kohn, W. Inhomogeneous electron gas. Phys. Rev 1964, 136, B864–B871. [Google Scholar]
- Kohn, W; Sham, LJ. Self-consistent equations including exchange and correlation effects. Phys. Rev 1965, 140, A1133–A1138. [Google Scholar]
- Lee, C; Yang, W; Parr, RG. Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar]
- Becke, AD. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys 1993, 98, 1372–1377. [Google Scholar]
- Stephens, PJ; Devlin, FJ; Chabalowski, CF; Frisch, MJ. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem 1994, 98, 11623–11627. [Google Scholar]
- Stratmann, RE; Scuseria, GE; Frisch, MJ. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys 1998, 109, 8218–8224. [Google Scholar]
- Bauernschmitt, R; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett 1996, 256, 454–464. [Google Scholar]
- Casida, ME; Jamorski, C; Casida, KC; Salahub, DR. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys 1998, 108, 4439–4449. [Google Scholar]
- Zhang, HY. Structure-activity relationships and rational design strategies for radical-scavenging antioxidants. Curr. Comput. Aided Drug Des 2005, 1, 257–273. [Google Scholar]
- Wright, JS; Johnson, ER; DiLabio, GA. Predicting the activity of phenolic antioxidants: theoretical methods, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc 2001, 123, 1173–1183. [Google Scholar]
- Ji, HF; Zhang, HY; Shen, L. A theoretical elucidation of radical-scavenging power of cyanindin. Nat. Prod. Commun 2006, 1, 229–235. [Google Scholar]
- Ji, HF; Zhang, HY; Shen, L. Proton dissociation is important to understanding structure-activity relationships of gallic acid antioxidants. Bioorg. Med. Chem. Lett 2006, 16, 4095–4098. [Google Scholar]
- Frisch, MJ; Trucks, GW; Schlegel, HB; Scuseria, GE; Robb, MA; Cheeseman, JR; Montgomery, JA; Vreven, T; Kudin, KN; Burant, JC; Millam, JM; Iyengar, SS; Tomasi, J; Barone, V; Mennucci, B; Cossi, M; Scalmani, G; Rega, N; Petersson, GA; Nakatsuji, H; Hada, M; Ehara, M; Toyota, K; Fukuda, R; Hasegawa, J; Ishida, M; Nakajima, T; Honda, Y; Kitao, O; Nakai, H; Klene, M; Li, X; Knox, JE; Hratchian, HP; Cross, JB; Adamo, C; Jaramillo, J; Gomperts, R; Stratmann, RE; Yazyev, O; Austin, AJ; Cammi, R; Pomelli, C; Ochterski, JW; Ayala, PY; Morokuma, K; Voth, GA; Salvador, P; Dannenberg, JJ; Zakrzewski, VG; Dapprich, S; Daniels, AD; Strain, MC; Farkas, O; Malick, DK; Rabuck, AD; Raghavachari, K; Foresman, JB; Ortiz, JV; Cui, Q; Baboul, AG; Clifford, S; Cioslowski, J; Stefanov, BB; Liu, G; Liashenko, A; Piskorz, P; Komaromi, I; Martin, RL; Fox, DJ; Keith, T; Al-Laham, MA; Peng, CY; Nanayakkara, A; Challacombe, M; Gill, PMW; Johnson, B; Chen, W; Wong, MW; Gonzalez, C; Pople, JA. Gaussian 03; Gaussian, Inc: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Haley, JL; Fitch, AN; Goyal, R; Lambert, C; Truscott, TG; Chacon, JN; Stirling, D; Schalch, W. The S1 and T1 energy levels of all-trans-.-carotene. J Chem Soc Chem Commun 1992, 1175–1176. [Google Scholar]
- Wang, C; Tauber, MJ. High-yield singlet fission in a zeaxanthin aggregate observed by picosecond resonance Raman spectroscopy. J. Am. Chem. Soc 2010, 132, 13988–13991. [Google Scholar]
- Hirayama, O; Nakamura, K; Hamada, S; Kobayasi, Y. Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids 1994, 29, 149–150. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ji, H.-F. Insight into the Strong Antioxidant Activity of Deinoxanthin, a Unique Carotenoid in Deinococcus Radiodurans. Int. J. Mol. Sci. 2010, 11, 4506-4510. https://doi.org/10.3390/ijms11114506
Ji H-F. Insight into the Strong Antioxidant Activity of Deinoxanthin, a Unique Carotenoid in Deinococcus Radiodurans. International Journal of Molecular Sciences. 2010; 11(11):4506-4510. https://doi.org/10.3390/ijms11114506
Chicago/Turabian StyleJi, Hong-Fang. 2010. "Insight into the Strong Antioxidant Activity of Deinoxanthin, a Unique Carotenoid in Deinococcus Radiodurans" International Journal of Molecular Sciences 11, no. 11: 4506-4510. https://doi.org/10.3390/ijms11114506




