Isoprostanes-Biomarkers of Lipid Peroxidation: Their Utility in Evaluating Oxidative Stress and Analysis
Abstract
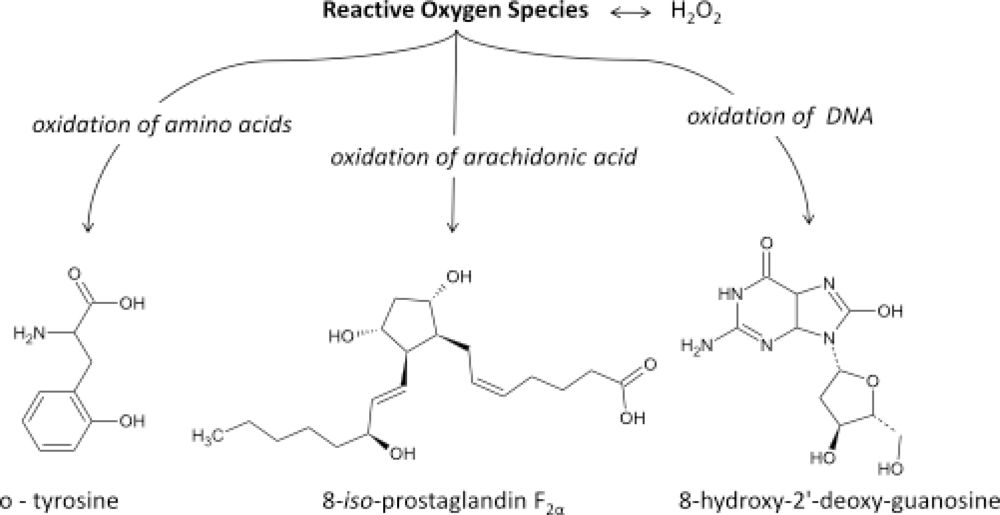
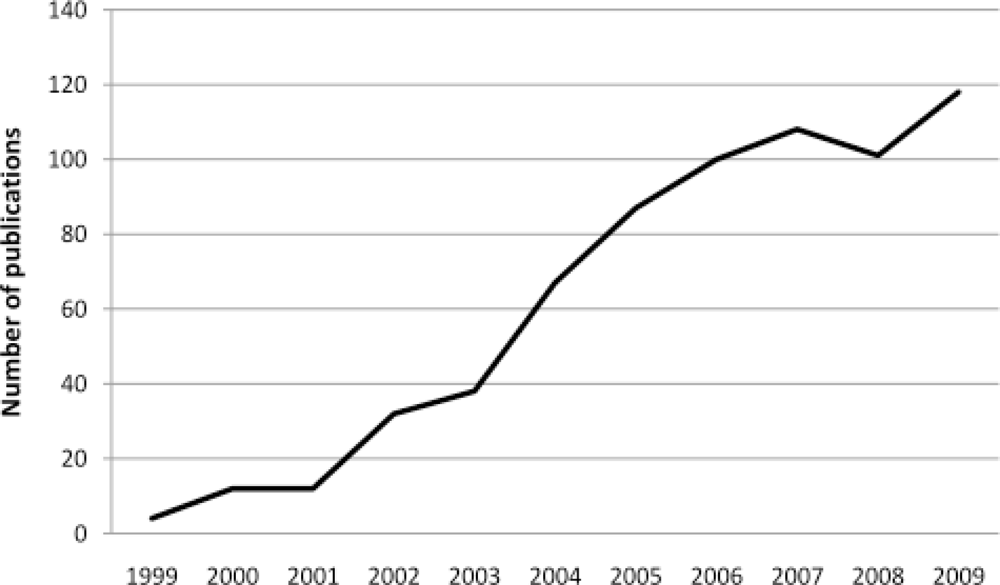
:1. Introduction
- It has now been established that measurement of IsoPs is the most reliable approach to the assessment of oxidative stress status in vivo, providing an important tool for exploring the role of oxidative stress in the pathogenesis of human disease;
- products of the IsoPs pathway have been found to exert potent biological actions and therefore may be pathophysiological mediators of disease.
2. Isoprostanes as Biomarkers of Diseases
2.1. Applications of Measurement of 8-IsoPGF2α in Lung Diseases
2.1.1. Allergy
2.1.2. Asbestos Exposure
2.1.3. Asthma
2.1.4. Chronic and Acute Exposures
2.1.5. Chronic Obstructive Pulmonary Disease—COPD
2.1.6. Idiopathic Pulmonary Fibrosis
2.1.7. Oxidative Stress in Smokers
2.1.8. Pneumoconioses
2.1.9. Sarcoidosis
2.1.10. Silicosis
2.1.11. Systemic Sclerosis
2.2. Standardization of EBC Measurement
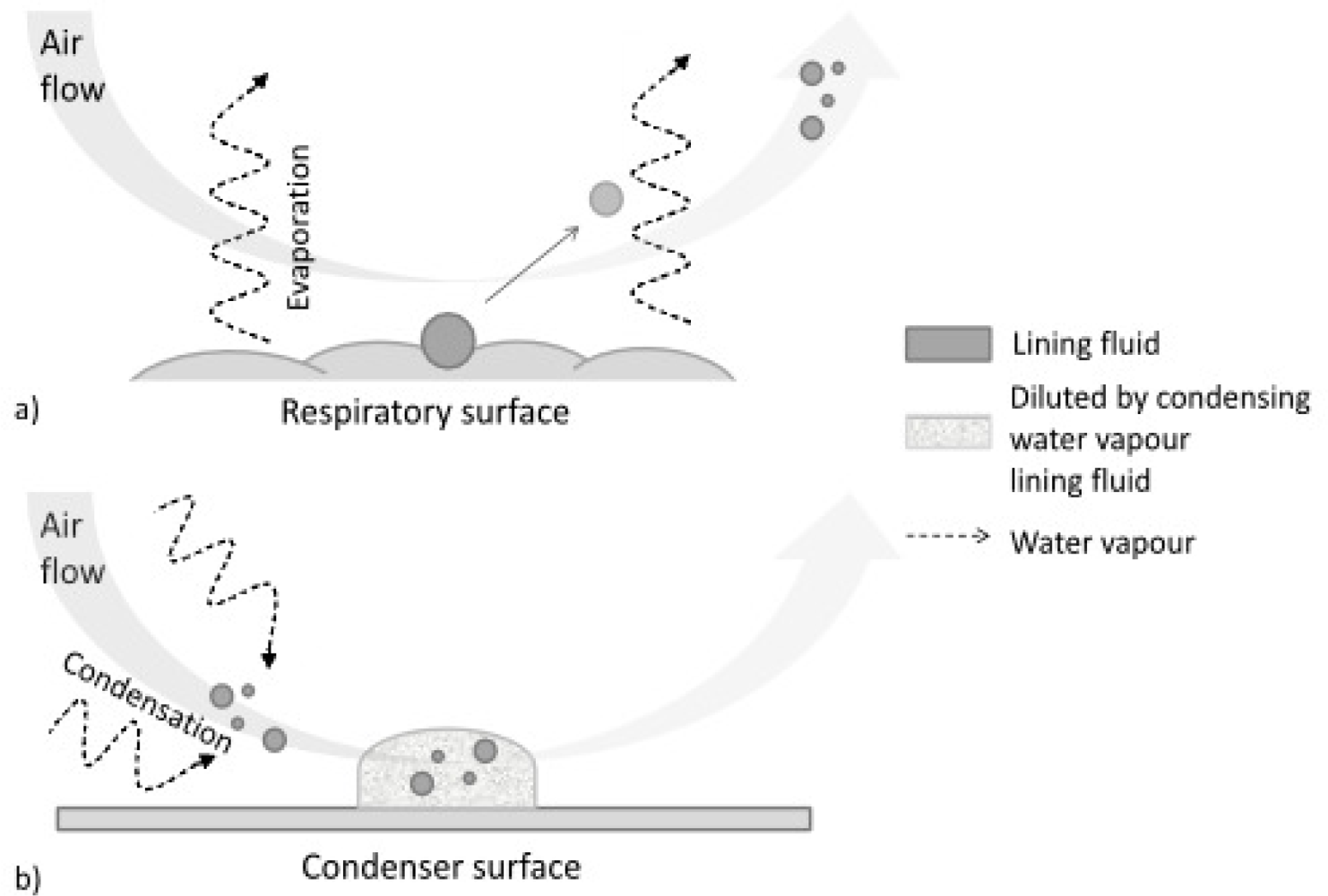
3. Composition of EBC Samples
- droplets of variable size (during normal tidal breathing, levels of aerosol particles range between 0.1 and 4 particles/cm3, the mean particle diameter is <0.3 μm [53–55]), aerosolized from the airway lining fluid—such particles, presumably reflecting the fluid itself, emanate from the mouth or endotracheal tube in exhaled air [56]; they serve as the only explanation for the presence of clearly non-volatile constituents in EBC such as sodium ions [57].
- water-soluble volatiles compounds (exhaled and absorbed in the condensing breath) and non-volatile constituents (compounds mostly derived from the airway lining fluid particles).
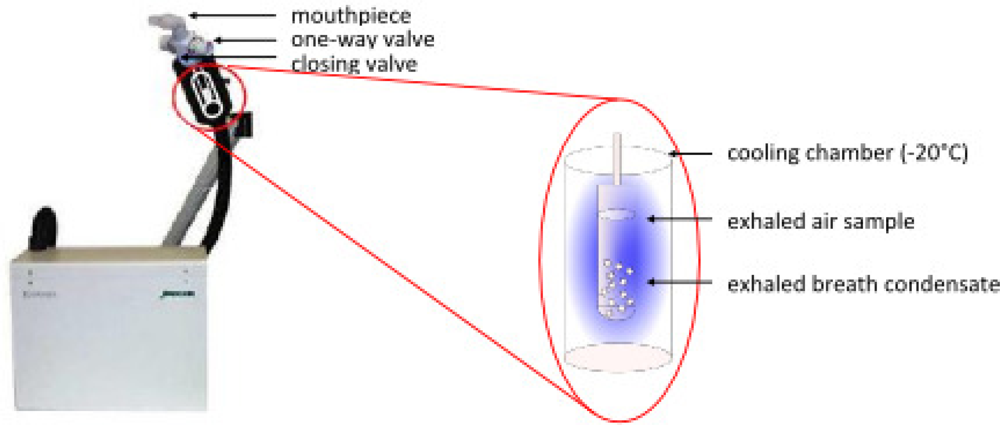
4. EBC Sample Collection
4.1. EcoScreen
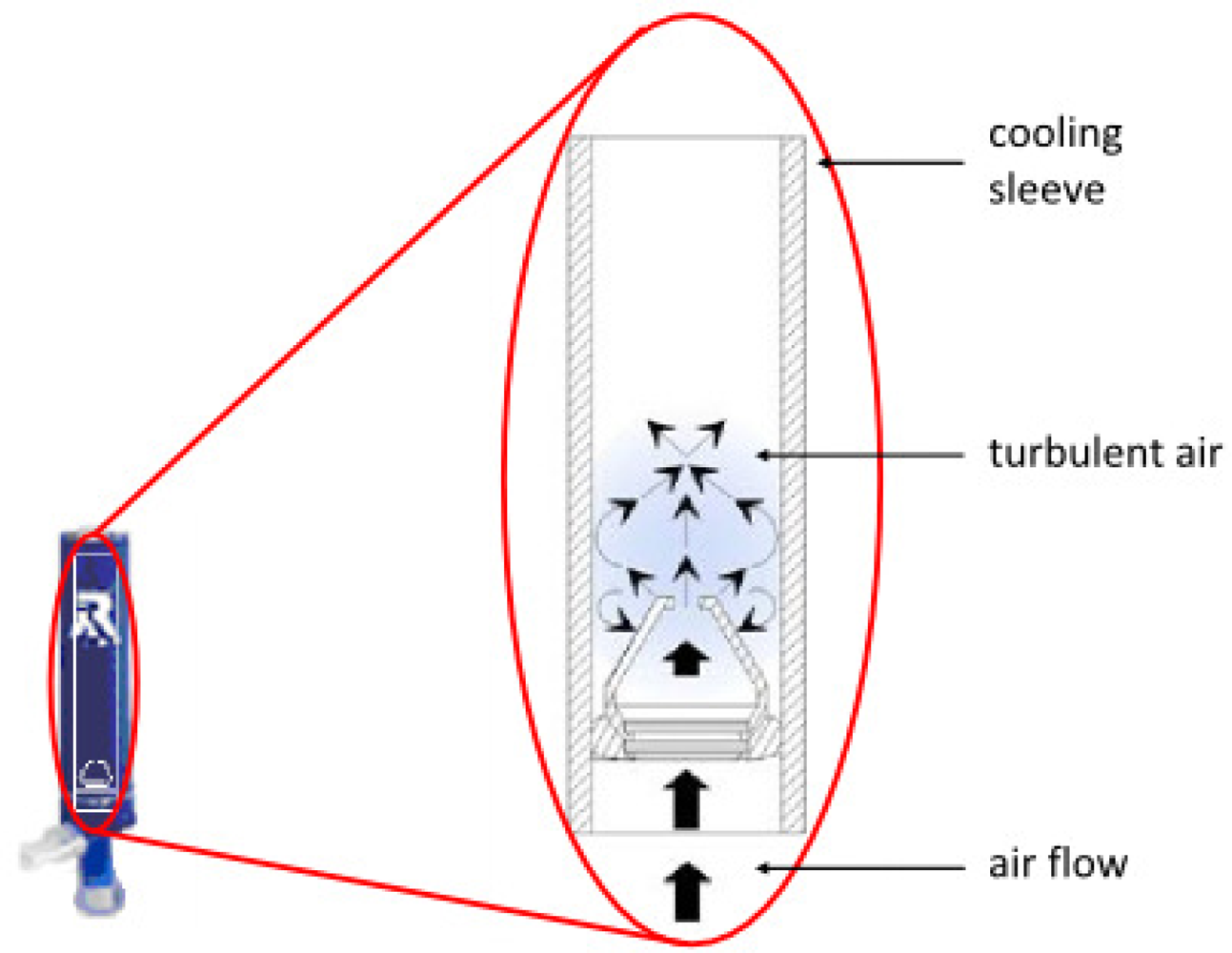
4.2. Rtube
4.3. Other Systems for EBC Collection
5. Salivary Contamination of EBC
6. Storage of EBC Samples
7. Sample Preparation
7.1. Lyophilization
7.2. Solid Phase Extraction
- Sample Pretreatment for interferent-laden samples (e.g., biological fluids)—dilute samples 1:1 with buffer. pH manipulation may be important in the case of ionizable compounds. A compound’s ionization state can drastically change its retention and elution characteristics on a given SPE sorbent. When an analyte is in its neutral form, it becomes more hydrophobic and retention strengthens under reversed-phase conditions. Adjusting the sample pH to 2 pH units above or below the compound’s pKa (depending on the functional group) will effectively neutralize the compound. Non-polar solvents (including methanol and isopropanol) prevent interaction between the compound and sorbent functional groups. To avoid clogging, it may be necessary to centrifuge, or pre-filter the sample prior to introducing it to the SPE phase.
- Conditioning/Equilibration wets or activates the bonded phases to ensure consistent interaction between the analyte and the sorbent functional groups. Reversed-phase sorbents are often conditioned with 1–2 tube volumes of a water-miscible solvent such as methanol or acetonitrile. Equilibration introduces a solution similar to the sample load in terms of solvent strength and pH in order to maximize retention. 1–2 tube volumes of buffer (used in sample pre-treatment) or water are good choices for reversed-phase equilibration.
- Sample Loading must be done at a consistent and reduced flow rate of ∼1–2 drops per second to ensure optimal retention.
- Washing of sample interferences that are often co-retained with target compounds during sample loading. A washing step is necessary to elute interferences without prematurely eluting target compounds. 5–20% methanol in water or sample pre-treatment buffer is typical for washing solvents.
- Elution can be performed with an organic solvent or solvent combination of sufficient non-polar character in order to prevent hydrophobic interactions between the analyte and sorbent functional groups. Example elution solvents are 1–2 volumes of methanol or acetonitrile. pH manipulation during elution can often improve recovery in the case of ionizable compounds. In their ionic form, basic and acidic compounds become more polar, attenuating reversed-phase interaction, so weaker elution solvents and/or reduced elution volumes can be used.
- Eluate Post-treatment is often necessary in, for example evaporation to dryness and reconstitution of SPE eluate in the mobile phase prior to LC analysis. GC analysis often requires further SPE eluate concentration, possible matrix exchange with a more volatile solvent and/or derivatization.
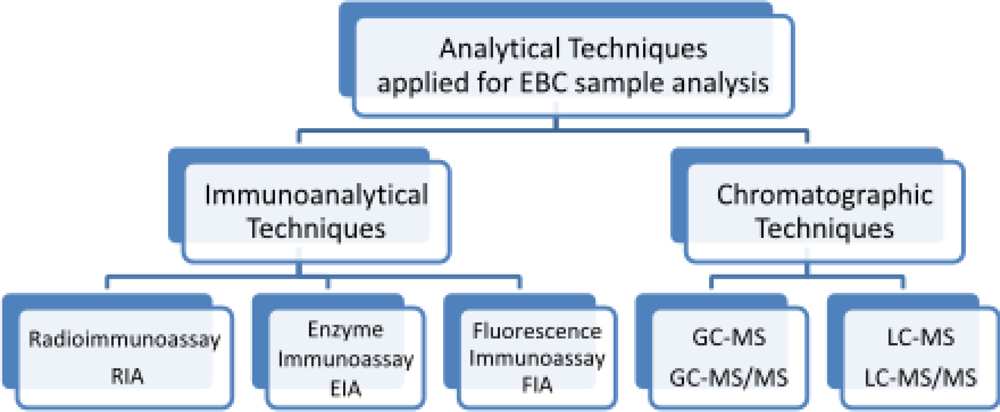
8. Determination of Isoprostanes in EBC Samples
8.1. Gas Chromatography with Mass Spectrometry and Tandem Mass Spectrometry Detection
8.2. Liquid Chromatography with Mass Spectrometry and Tandem Mass Spectrometry Detection
9. Trends and Future Directions
10. Conclusion
Acknowledgments
References
- Montuschi, P; Barnes, PJ; Roberts, LJ, II. Isoprostanes: Markers and mediators of oxidative stress. FASEB J 2004, 18, 1791–1800. [Google Scholar]
- Płacik, B; Nofer, TW; Wąsowicz, W. F2-isoprostanes biomarkers of lipid peroxidation: Their utility in evaluation of oxidative stress induced by toxic agents. Int. J. Occup. Med. Environ. Health 2002, 15, 19–27. [Google Scholar]
- Rokach, J; Khanapure, SP; Hwang, SW; Adiyaman, M; Lawson, JA; FitzGerald, GA. Isoprostanes: A perspectives. Prostaglandins 1997, 54, 823–851. [Google Scholar]
- Cracowski, JL; Durand, T; Bessard, G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol. Sci 2002, 23, 360–366. [Google Scholar]
- Comporti, M; Signorini, C; Arezzini, B; Vecchio, D; Monaco, B; Gardi, C. F2-isoprostanes are not just markers of oxidative stress. Free Radical Bio. Med 2008, 44, 247–256. [Google Scholar]
- Bohnstedt, KC; Karlberg, B; Wahlund, L-O; Jönhagen, ME; Basun, H; Schmidt, S. Determination of isoprostanes in urine samples from Alzheimer patients using porous graphitic carbon liquid. J. Chromatogr. B 2003, 796, 11–19. [Google Scholar]
- Harman, SM; Liang, L; Tsitouras, PD; Gucciardo, F; Heward, CB; Reaven, PD; Ping, W; Ahmed, A; Cutler, RG. Urinary Excretion of Three Nucleic Acid Oxidation Adducts and Isoprostane F2α Measured by Liquid Chromatography-Mass Spectrometry in Smokers, Ex-smokers, and Nonsmokers. Free Radical Bio. Med 2003, 35, 1301–1309. [Google Scholar]
- Ohashi, N; Yoshikawa, M. Rapid and sensitive quantification of 8-isoprostaglandin F in 2a human plasma and urine by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. B 2000, 746, 17–24. [Google Scholar]
- Saenger, AK; Laha, TJ; Edenfield, MJ; Sadrzadeh, SMH. Quantification of urinary 8-iso-PGF2α using liquid chromatography-tandem mass spectrometry and association with elevated troponin levels. Clin. Biochem 2007, 40, 1297–1304. [Google Scholar]
- Taylor, AW; Bruno, RS; Frei, B; Traber, MG. Benefits of prolonged gradient separation for high-performance liquid chromatography-tandem mass spectrometry quantitation of plasma total 15-series F2-isoprostanes. Anal. Biochem 2006, 350, 41–51. [Google Scholar]
- Greco, A; Minghetti, L; Puopolo, M; Pietrobon, B; Franzoi, M; Chiandetti, L; Suppiej, A. Plasma levels of 15-F2t-isoprostane in newborn infants are affected by mode of delivery. Clin. Biochem 2007, 40, 1420–1422. [Google Scholar]
- Montine, TJ; Markesbery, WR; Morrow, JD; Robert, LJ, II. Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Ann. Neurol 1998, 44, 410–413. [Google Scholar]
- Shahid, SK; Kharitonov, SA; Wilson, NM; Bush, A; Barnes, PJ. Exhaled 8-isoprostane in childhood asthma. Respir. Res 2005, 6, 1–6. [Google Scholar]
- Syslová, K; Kačer, P; Kuzma, M; Klusáčková, P; Fenclová, Z; Lebedová, J; Pelclová, D. Determination of 8-iso-prostaglandin F2 in exhaled breath condensate using combination of immunoseparation and LC–ESI-MS/MS. J. Chromatogr. B 2008, 867, 8–14. [Google Scholar]
- Psathakis, K; Mermigkis, D; Papatheodorou, G; Loukides, S; Panagou, P; Siafakas, VPM; Bouros, D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur. J. Clin. Invest 2006, 36, 362–367. [Google Scholar]
- Peclová, D; Fenclová, Z; Kačer, P; Kuzma, M; Navrátil, T; Lebedová, J. Increased 8-isoprostane, a Marker of Oxidative Stress in Exhaled Breath Condensate in Subjects with Asbestos Exposure. Ind. Health 2008, 46, 484–489. [Google Scholar]
- Montuschi, P. LC-MS-MS analysis of leukotriene B4 and other eicosanoids in exhaled breath condensate for assessing lung inflamation. J. Chromatogr. B 2009, 877, 1272–1280. [Google Scholar]
- Xie, J; Zhang, Q; Zhong, N; Lai, K. BAL Fluid 8-Isoprostane Concentrations in Eosinophilic Bronchitis and Asthma. J. Asthma 2009, 46, 712–715. [Google Scholar]
- Longini, M; Perrone, S; Kenanidis, A; Vezzosi, P; Marzocchi, B; Petragli, F; Centini, G; Buonocore, G. Isoprostanes in amniotic fluid: A predictive marker for fetal growth restriction in pregnancy. Free Radical Bio. Med 2005, 38, 1537–1541. [Google Scholar]
- Morrow, JD; Roberts, LJ. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol 1999, 300, 3–12. [Google Scholar]
- Patrignani, P; Tacconelli, S. Isoprostanes and other markers of peroxidation in atherosclerosis. Biomarkers 2005, 10, 24–29. [Google Scholar]
- Miekisch, W; Schubert, JK. From highly sophisticated analytical techniques to life-saving diagnostics: Technical developments in breathanalysis. Trends Anal. Chem 2006, 25, 665–673. [Google Scholar]
- Hunt, J; Byrns, RE; Ignarro, LJ; Gaston, B. Condensed expirate nitrite as a home marker for acute asthma. Lancet 1995, 346, 1235–1236. [Google Scholar]
- Praticò, D; Lawsona, JA; Rokach, J; FitzGerald, GA. The isoprostanes in biology and medicine. Trends Endocrinol. Metab 2001, 12, 243–247. [Google Scholar]
- Samitas, K; Chorianopoulos, D; Vittorakis, S; Zervas, E; Economidou, E; Papatheodorou, G; Loukides, S; Gaga, M. Exhaled cysteinyl-leukotrienes and 8-isoprostane in patients with asthma and their relation to clinical severity. Respir. Med 2009, 103, 750–753. [Google Scholar]
- Do, R; Bartlett, KH; Dimich-Ward, H; Chu, W; Kennedy, SM. Biomarkers of Airway Acidity and Oxidative Stress in Exhaled Breath Condensate from Grain Workers. Am. J. Respir. Crit. Care Med 2008, 178, 1048–1054. [Google Scholar]
- Montuschi, P. Exhaled breath condensate analysis in patients with COPD. Clin. Chim. Acta 2005, 356, 22–34. [Google Scholar]
- Carpenter, CT; Price, PV; Christman, BW. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest 1998, 114, 1653–1659. [Google Scholar]
- Petrosyan, M; Perraki, E; Simoes, D; Koutsourelakis, I; Vagiakis, E; Roussos, C; Gratziou, C. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath 2008, 12, 207–215. [Google Scholar]
- Antczak, A; Montuschi, P; Kharitonov, SA; Gorski, P; Barnes, PJ. Increased exhaled cysteinyl-leukotrienes and 8-isoprostane in aspirin-induced asthma. Am. J. Respir. Crit. Care Med 2002, 166, 301–306. [Google Scholar]
- Baraldi, E; Ghiro, L; Piovan, V. Increased exhaled 8-isoprostane in childhood asthma. Chest 2003, 124, 25–31. [Google Scholar]
- Baraldi, E; Carraro, S; Alinovi, R; Pesci, A; Ghiro, L; Bodini, A; Piacentini, G; Zacchello, F; Zanconato, S. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbation. Thorax 2003, 58, 505–509. [Google Scholar]
- Zanconato, S; Carraro, S; Corradi, M; Alinovi, R; Pasquale, MF; Piacentini, G; Zacchello, F; Baraldi, E. Leukotrienes and 8-isoprostane in exhaled breath condensate of children with stable and unstable asthma. J. Allergy Clin. Immunol 2004, 113, 257–263. [Google Scholar]
- Biernacki, WA; Kharitonov, SA; Barnes, PJ. Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbations of COPD. Thorax 2003, 58, 294–298. [Google Scholar]
- Montuschi, P; Ciabattoni, G; Paredi, P; Pantelidis, P; Bois, RMd; Kharitonov, SA; Barnes, PJ. 8-Isoprostane as a Biomarker of Oxidative Stress in Interstitial Lung Diseases. Am. J. Respir. Crit. Care Med 1998, 58, 1524–1527. [Google Scholar]
- Lucidi, V; Ciabattoni, G; Bella, S; Barnes, PJ; Montuschi, P. Exhaled 8-isoprostane and prostaglandin E2 in patients with stable and unstable cystic fibrosis. Free Radical Bio. Med 2008, 45, 913–919. [Google Scholar]
- Psathakis, K; Papatheodorou, G; Plataki, M. 8-Isoprostane, a marker of oxidative stress, is increased in the expired breath condensate of patients with pulmonary sarcoidosis. Chest 2004, 125, 1005–1011. [Google Scholar]
- Carpagnano, GE; Kharitonov, SA; Resta, O; Foschino-Barbaro, MP; Gramiccioni, E; Barnes, PJ. Increased 8-Isoprostane and Interleukin-6 in Breath Condensate of Obstructive Sleep Apnea Patients. Chest 2002, 122, 1162–1167. [Google Scholar]
- Alfaro, MF; Walby, WF; Adams, WC; Schelegle, ES. Breath condensate levels of 8-isoprostane and leukotriene B4 after ozone inhalation are greater in sensitive versus nonsensitive subjects. Exp. Lung Res 2007, 33, 115–133. [Google Scholar]
- MontuschiI, P; Barnes, PJ; Roberts, LJ, II. Isoprostanes: Markers and mediators of oxidative stress. FASEB J 2004, 18, 1791–1800. [Google Scholar]
- Montuschi, P; Barnes, PJ; Ciabattoni, G. Advanced Protocols in Oxidative Stress II; Humana Press: New York, NY, USA, 2009; p. 594. [Google Scholar]
- Tanou, K; Koutsokera, A; Kiropoulos, TS; Maniatiw, M; Papaioannou, AI; Georga, K; Zarogiannis, S; Gourgoulianis, KI; Kostikas, K. Inflammatory and oxidative stress biomarkers in allergic rhinitis: The effect of smoking. Clin. Exp. Allergy 2009, 39, 345–353. [Google Scholar]
- Gratziou, C; Rovina, N; Makris, M; Simoes, DC; Papapetropoulos, A; Roussos, C. Breath markers of oxidative stress and airway inflammation in seasonal allergic rhinitis. Int. J. Immunopathol. Pharmacol 2008, 21, 949–957. [Google Scholar]
- Chow, S; Campbell, C; Sandrini, A; Thomas, PS; Johnson, AR; Yates, DH. Exhaled breath condensate biomarkers in asbestos-related lung disorders. Respir. Med 2009, 103, 1091–1097. [Google Scholar]
- Carraro, S; Cogo, PE; Isak, I; Simonato, M; Corradi, M; Carnielli, VP; Baraldi, E. EIA and GC-MS analysis of 8-isoprostane in EBC of children with problematic asthma. Eur. Respir. J 2010, 35, 1364–1369. [Google Scholar]
- Makris, D; Paraskakis, E; Korakas, P; Karagiannakis, E; Sourvinos, G; Siafakas, NM; Tzanakis, N. Exhaled Breath Condensate 8-Isoprostane, Clinical Parameters, Radiological Indices and Airway Inflammation in COPD. Respiration 2008, 75, 138–144. [Google Scholar]
- Hoydonck, PGAV; Wuyts, WA; Vanaudenaerde, BM; Schouten, EG; Dupont, LJ; Temme, EHM. Quantitative analysis of 8-isoprostane and hydrogen peroxide in exhaled breath condensate. Eur. Respir. J 2004, 23, 189–192. [Google Scholar]
- Pelclová, D; Fenclová, Z; Krmenciková, M; Navratil, T; Kuzma, M; Kacer, P. Exhaled breath condensate markers of oxidant stress in patients with pneumoconioses. Chest 2008, 6001. [Google Scholar]
- Piotrowski, WJ; Kurmanowska, Z; Antczak, A; Marczak, J; Górski, P. Exhaled 8-isoprostane as a prognostic marker in sarcoidosis. A short term follow-up. BMC Pulm. Med 2010, 10, 1–7. [Google Scholar]
- Pelclová, D; Fenclová, Z; Kacer, P; Navrátil, T; Kuzma, M; Lebedová, JK; Klusácková, P. 8-isoprostane and leukotrienes in exhaled breath condensate in Czech subjects with silicosis. Ind. Health 2007, 45, 766–774. [Google Scholar]
- Tufvesson, E; Bozovic, G; Hesselstrand, R; Bjermer, L; Scheja, A; Wuttge, DM. Increased cysteinyl-leukotrienes and 8-isoprostane in exhaled breath condensate from systemic sclerosis patients. Rheumatology 2010, 49, 2322–2326. [Google Scholar]
- Horvath, I; Hunt, J; Barnes, PJ. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur. Respir. J 2005, 26, 523–548. [Google Scholar]
- Fritter, D; Knobler, CM. Experiments and simulation of the growth of droplets on a surface (breath figures). Phys. Rev. A 1991, 43, 2858–2869. [Google Scholar]
- Mutlu, GM; Garey, KW; Robbins, RA; Danziger, LH; Rubinstein, I. Collection and Analysis of Exhaled Breath Condensate in Humans. Am. J. Respir. Crit. Care Med 2001, 164, 731–737. [Google Scholar]
- Fairchild, CI; Stampfer, JF. Particle concentration in exhaled breath. Am. Ind. Hyg. Assoc. J 1987, 48, 948–949. [Google Scholar]
- Papineni, RS; Rosenthal, FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med 1997, 10, 105–116. [Google Scholar]
- Zacharasiewicz, A; Wilson, N; Lex, C; Li, A; Kemp, M; Donovan, J; Hooper, J; Kharitonov, SA; Bush, A. Repeatability of sodium and chloride in exhaled breath condensates. Pediatr. Pulmonol 2004, 37, 273–275. [Google Scholar]
- Hunt, J. Exhaled breath condensate: An evolving tool for noninvasive evaluation of lung disease. J. Allergy Clin. Immunol 2002, 110, 28–34. [Google Scholar]
- Lema, JBD; González, M; Vigil, L; Casan, P. Exhaled Breath Condensate: Standardized Collection of Samples From Healthy Volunteer. Arch. Bronconeumol 2005, 41, 584–586. [Google Scholar]
- Sznajder, JI; Fraiman, A; Hall, JB; Sanders, W; Schmidt, G; Crawford, G; Nahum, A; Factor, P; Wood, LD. Increased hydrogen peroxide in the expired breath of patients with acute hypoxemic respiratory failure. Chest 1989, 96, 606–612. [Google Scholar]
- Rosias, PP; Robroeks, CM; Niemarkt, HJ; Kester, AD; Vernooy, JH; Suykerbuyk, J; Teunissen, J; Heynens, J; Hendriks, HJ; Jobsis, Q; Dompeling, E. Breath condenser coatings affect measurement of biomarkers in exhaled breath condensate. Eur. Respir. J 2006, 28, 1036–1041. [Google Scholar]
- Hoffmeyer, F; Raulf-Heimsoth, M; Harth, V; Bünger, J; Brüning, T. Comparative analysis of selected exhaled breath biomarkers obtained with two different temperature-controlled devices. BMC Pulm. Med 2009, 9, 1–10. [Google Scholar]
- Carpagnano, GE; Resta, O; Foschino-Barbaro, MP; Spanevello, A; Stefano, A; Gioia, GD; Serviddio, G; Gramiccioni, E. Exhaled Interleukine-6 and 8-isoprostane in chronic obstructive pulmonary disease: effect of carbocysteine lysine salt monohydrate (SCMC-Lys). Eur. J. Pharmacol 2004, 505, 169–175. [Google Scholar] [Green Version]
- Moloney, ED; Mumby, SE; Gajdocsi, R; Cranshaw, JH; Kharitonov, SA; Quinlan, GJ; Griffiths, MJ. Exhaled Breath Condensate Detects Markers of Pulmonary Inflammation after Cardiothoracic Surgery. J. Respir. Crit. Care Med 2004, 169, 64–69. [Google Scholar]
- Komakula, S; Khatri, S; Mermis, J; Savill, S; Haque, S; Rojas, M; Brown, L; Teague, GW; Holguin, F. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir. Res 2007, 8, 1–10. [Google Scholar]
- Ngamtrakulpanit, L; Yu, Y; Adjei, A; Amoah, G; Gaston, B; Hunt, JF. Identification of Intrinsic Airway Acidification in Pulmonary Tuberculosis. Global J. Health Sci 2010, 2, 106–110. [Google Scholar]
- Izquierdo, JL; Almonacid, C; Parra, T; Pérez, J. Systemic and lung inflammation in 2 phenotypes of chronic obstructive pulmonary disease. Arch. Bronconeumol 2006, 42, 332–337. [Google Scholar]
- Almonacid, C; Izquierdo, JL; Parra, T; Pérez, J. El pH medido en el condensado de aire exhalado se correlaciona con cambios en los marcadores inflamatorios en los pacientes con. EPOC. Rev. Patol Respir 2007, 10, 69–75. [Google Scholar]
- Corradi, M. Exhaled breath condensate in paediatric pulmonology. Pneumologia Pediatrica 2006, 24, 12–19. [Google Scholar]
- Nowak, D; Kobus, M; Mokwiński, M. Urządzenie do zbierania kondensatu pary wodnej wydychanego powietrza. PL Patent 192973. 2003.
- Liu, J; Conrad, DH; Chow, S; Tran, VH; Yates, DH; Thomas, PS. Collection devices influence the constituents of exhaled breath condensate. Eur. Respir. J 2007, 30, 807–808. [Google Scholar]
- Koczulla, R; Dragonieri, S; Schot, R; Bals, R; Gauw, SA; Vogelmeier, C; Rabe, KF; Sterk, PJ; Hiemstra, PS. Comparison of exhaled breath condensate pH using two commercially available devices in healthy controls, asthma and COPD patients. Respir. Res 2009, 10, 1–8. [Google Scholar]
- ValenzuelaI, OFA; Encina, MPS. Design and evaluation of a device for collecting exhaled breath condensate. J. Bras. Pneumol 2009, 35, 69–72. [Google Scholar]
- Effros, RM; Dunning, MB, III; Biller, J; Shaker, R. The promise and perils of exhaled breath condensates. Am. J. Physiol. Lung Cell Mol. Physiol 2004, 287, L1073–L1080. [Google Scholar]
- Rosias, PPR; Robroeks, CM; Kant, KDVD; Rijkers, GT; Zimmermann, LJ; Schayck, CPV; Heynens, JW; Jobsis, Q; Dompeling, E. Feasibility of a new method to collect exhaled breath condensate in pre-school children. Pediatr. Allergy Immunol 2010, 21, 235–244. [Google Scholar]
- Gaber, F; Acevedo, F; Delin, I; Sundblad, BM; Palmberg, L; Larsson, K; KumLin, M; Dahlen, SE. Saliva is one likely source of leukotriene B4 in exhaled breath condensate. Eur. Respir. J 2006, 28, 1229–1235. [Google Scholar]
- Effros, RM; Peterson, B; Casaburi, R; Su, J; Dunning, M; Torday, J; Biller, J; Shaker, R. Epithelial lining fluid solute concentrations in chronic obstructive lung disease patients and normal subjects. J. Appl. Physiol 2005, 99, 1286–1292. [Google Scholar]
- Huszar, E; Vass, G; Vizi, E; Csoma, Z; Barat, E; Molnar, VG; Herjavecz, I; Horvath, I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur. Respir. J 2002, 20, 1393–1398. [Google Scholar]
- Kharitonov, SA; Barnes, PJ. Exhaled Markers of Pulmonary Disease. Am. J. Respir. Crit. Care Med 2001, 163, 1693–1722. [Google Scholar]
- Griese, M; Noss, J; Bredow, CV. Protein pattern of exhaled breath condensate and saliva. Proteomics 2002, 2, 690–696. [Google Scholar]
- Reinhold, P; Knobloch, H. Exhaled breath condensate: Lessons learned from veterinary medicine. J. Breath Res 2010, 4, 017001. [Google Scholar]
- Vaughan, J; Ngamtrakulpanit, L; Pajewski, TN; Turner, R; Nguyen, T-A; Smith, A; Urban, P; Hom, S; Gaston, B; Hunt, J. Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur. Respir. J 2003, 22, 889–894. [Google Scholar]
- Svensson, S; Olin, A-C; Larstad, M; Ljungkvist, G; Toren, K. Determination of hydrogen peroxide in exhaled breath condensate by flow injection analysis with fluorescence detection. J. Chromatogr. B 2004, 809, 199–203. [Google Scholar]
- Gessner, C; Scheibe, R; Wotzel, M; Hammerschmidt, S; Kuhn, H; Engelmann, L; Hoheisel, G; Gillissen, A; Sack, U; Wirtz, H. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respir. Med 2005, 99, 1229–1240. [Google Scholar]
- Syslová, K; Kačer, P; Kuzma, M; Pankrácová, A; Fenclová, Z; Vlčková, Š; Lebedová, J; Pelclová, D. LC-ESI-MS/MS method for oxidative stress multimarker screening in the exhaled breath condensate of asbestosis/silicosis patients. J. Breath Res 2010, 4, 017104. [Google Scholar]
- Wells, K; Vaughan, J; Pajewski, TN; Hom, S; Ngamtrakulpanit, L; Smith, A; Ngujen, A; Turner, R. Exhaled breath condensate pH assays are not influenced by oral amonia. Thorax 2005, 60, 27–31. [Google Scholar]
- Goldoni, M; Caglieri, A; Andreoli, R; Poli, D; Manini, P; Vettori, MV; Mutti, A. Influence of condensation temperature on selected exhaled breath parameters. BMC Pulm. Med 2005, 5, 1–9. [Google Scholar]
- White, DC; Geyer, R; Cantu, J; Jo, S-C; Peacock, AD; Saxton, AM; Mani, S; Jett, M; Moss, OR. Feasibility of assessment of regulatory lipids in breath condensate as potential presymptomatic harbingers of pulmonary pathobiology. J. Microbiol. Methods 2005, 62, 293–302. [Google Scholar]
- Syslová, K; Kačer, P; Kuzma, M; Najmanová, V; Fenclová, Z; Vlčková, Š; Lebedová, J; Pelclová, D. Rapid and easy method for monitoring oxidative stress markers in body fluids of patients with asbestos or silica-induced lung diseases. J. Chromatogr. B 2009, 877, 2477–2486. [Google Scholar]
- Montuschi, P; Ragazzoni, E; Valente, S; Corbo, G; Mondino, C; Ciappi, G; Ciabattoni, G. Validation of 8-isoprostane and prostaglandin E2 measurements. Inflamm. Res 2003, 52, 502–507. [Google Scholar]
- Montuschi, P; Mondino, C; Koch, P; Barnes, PJ; Ciabattoni, G. Effects of a leukotriene receptor antagonist on exhaled leukotriene E4 and prostanoids in children with asthma. J. Allergy Clin. Immunol 2006, 118, 347–353. [Google Scholar]
- Piotrowski, WJ; Antczak, A; Marczak, J; Nawrocka, A; Kurmanowska, Z; Górski, P. Eicosanoids in Exhaled Breath Condensate and BAL fluid of patients with sarkoidosis. Chest 2007, 132, 589–596. [Google Scholar]
- Li, Y; Chongsuvivatwong, V; Geater, A; Liu, A. Exhaled breath condensate cytokine level as a diagnostic tool for obstructive sleep apnea syndrome. Sleep Med 2009, 10, 95–103. [Google Scholar]
- Ko, FWS; Lau, CYK; Leung, TF; Wong, GWK; Lam, CWK; Hui, DSC. Exhaled breath condensate levels of 8-isoprostane, growth related oncogene a and monocyte chemoattractant protein-1 in patients with chonic obstructive pulmonary disease. Respir. Med 2006, 100, 630–638. [Google Scholar]
- Dalaveris, E; Kerenidi, T; Katsabeki-Katsafli, A; Kiropoulos, T; Tanou, K; Gourgoulianis, KI; Kostikas, K. VEGF, TNFa and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer 2009, 64, 219–225. [Google Scholar]
- Gonzalez-Reche, LM; Schaefer, D; Göen, T; Kraus, T. Validity Assessment for the Results of Three Inflammatory Markers in Exhaled Breath Condensate—A Pilot Study. Chromatographia 2009, 70, 1387–1392. [Google Scholar]
- Holz, O. New Perspectives in Monitoring Lung Inflammation: Analysis of Exhaled Breath Condensate; CRC Press LLC: Washington, DC, USA, 2005. [Google Scholar]
- Lee, C-YJ; Huang, SH; Jenner, AM; Halliwell, B. Measurement of F2-isoprostanes, hydroxyeicosatetraenoic products, and oxysterols from a single plasma sample. Free Radical Bio. Med 2008, 44, 1314–1322. [Google Scholar]
- Limb, SL; Hubbard, WC; Wood, RA; Adkinson, N, Jr. Asthma Biomarkers in Exhaled Breath Condensate (EBC). J. Allergy Clin. Immunol 2006, 117, 194. [Google Scholar]
- Montuschi, P; Barnes, PJ. Analysis of exhaled breath condensate for monitoring airway inflammation. Trends Pharmacol. Sci 2002, 23, 232–237. [Google Scholar]
- Grob, NM; Aytekin, M; Dweik, RA. Biomarkers in exhaled breath condensate: A review of collection, processing and analysis. J. Breath Res 2008, 2, 037004. [Google Scholar]
- Carraro, S; Rezzi, S; Reniero, F; Héberger, K; Giordano, G; Zanconato, S; Guillou, C; Baraldi, E. Metabolomics Applied to Exhaled Breath Condensate in Childhood Asthma. Am. J. Respir. Crit. Care Med 2007, 175, 986–990. [Google Scholar]
- Laurentiis, GD; Paris, D; Melck, D; Maniscalco, M; Marsico, S; Corso, G; Motta, A; Sofia, M. Metabonomic analysis of Exhaled Breath Condensate in adults by nuclear magnetic resonance spectroscopy. Eur. Respir. J 2008, 32, 1175–1183. [Google Scholar]



| EBC Collection System (Manufacturer) | Advantages | Disadvantages | References |
|---|---|---|---|
| ECoScreen I and ECoScreen II (Viasys, U.S., Europe) | The most commonly applied EBC collection system. More often used in European countries. There is an optional package for determining the total exhaled volume. | Not readily portable. Cleaning between patients may need to be extensive to abide by standard respiratory care practices. No way of controlling condensation temperature (Eco1). | [13,29,44,49,62–63] |
| Rtube (Respiratory Research, U.S.) | More total EBC collections than other systems. Multiple collections can be performed concurrently. Most commonly applied in North American centers. No cleaning between patients is necessary. Portable. Can be prepared for use in a standard freezer. | Choice and maintenance of set condensing temperature requires an optional cooling unit, otherwise the condensation temperature is chosen by cooling the sleeve preparation temperature and rises during collection. | [64–66] |
| Anacon (Biostec, Valencia, Spain) | Temperature of collection can be controlled. Designed for use on ventilated patients | Only a few publications are available. | [67,68] |
| TurboDeccs (ItalChill, Parma, Italy) | Has both non-disposable and disposable portions. Controllable collection temperature. Moderately portable. Readily cleanable because components are disposable. | Very few publications exist. Only one collection at a time is possible. | [69] |
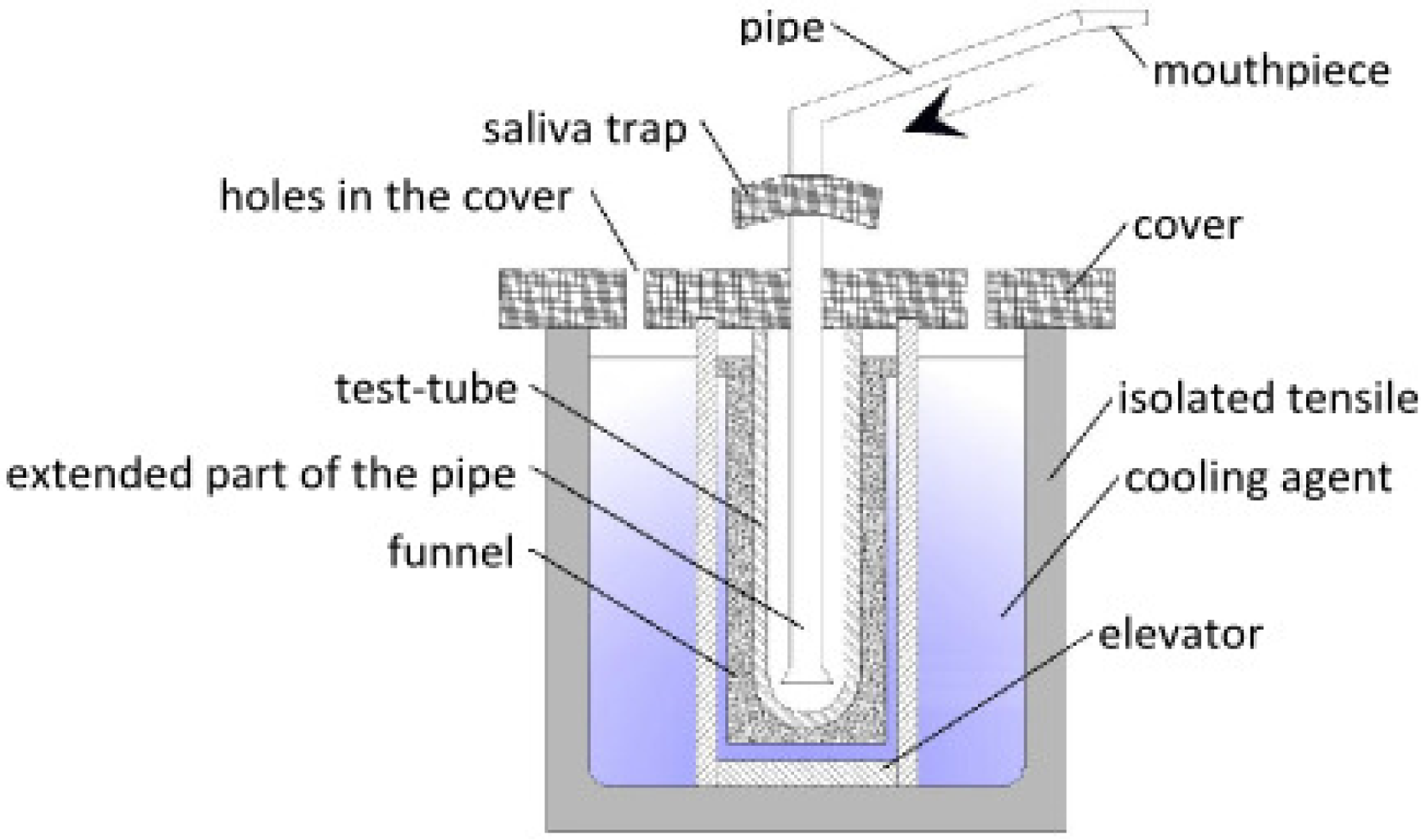
| Polish Patent | Constant maximum contact between the test-tube and the cooling agent. Possibility of inserting different sizes of test tubes. | Dismantling and cleaning after use is necessary. | [70] |
| Analytical Technique | Sample Preparation | LOD | Ref. |
|---|---|---|---|
| RIA | standard curves obtained using phosphate buffer 0.025 mol/L, pH 7.5, more similar to EBC matrix | 10 pg/mL | [91] |
| samples stored at −80 °C before analysis | 10 pg/mL | [36] | |
| EIA | after sampling no further preparation of the sample is necessary, samples stored at −80 °C before analysis | 4–5 g/mL | [25,46,63, 92–95] |
| SPE of pooleed sample (35.5 mL), HPLC purification | 10 pg/mL | [90] | |
| LC-MS/MS | samples pretreated with 50 μL of immunoaffinity sorbent for 60 min | 1 pg/mL | [17] |
| after collection sample spiked with 8-isoPGF2α-d4, stored at −80 °C, lyophilization, redissolved in 50 μL of water/methanol (1:1) | 8 pg/mL | [85] | |
| sample spiked with 8-isoPGF2α-d4, pretreated with 50 μL of immunoaffinity sorbent for 60min at 20 °C, centrifuged (3000 rpm for 5 min), immunoaffinity sorbent flushed twice with water, elution with methanol, methanol evaporated, redissolved in 50 μL of mobile phase | 1 pg/mL | [14] | |
| LC-MS | immunoaffinity separation | 1 pg/mL | [16] |
| Specimen | Determined Analyte | Column | Eluents | Ref. |
|---|---|---|---|---|
| EBC | 8-iso-PGF2α | Hypercarb Thermo 100 mm × 2.1 mm × 5 μm | A: acetonitrile B: water (pH 11) isocratic elution A:B (7:3) flow rate 250 μL/min | [14,17] |
| EBC | 8-iso-PGF2α o-Tyr 8-OHdG | Hypercarb Thermo 100 mm × 2.1 mm × 5 μm | A: aqueous solution of ammonium hydroxide pH 10.5 B: methanol:acetonitrile 60:40 (v/v) + 0.1% of ammonium hydroxide gradient elution | [85] |
| Urine Plasma | 8-iso-PGF2α | Symmetry C8 150 mm × 3.9 mm × 5 μm | A: 0.1% acetic acid (pH 3) B: acetonitrile isocratic elution A:B (7:3) | [8] |
| Urine | 8-iso-PGF2α | YMC ODS-AQ 50 mm × 2.0 mm × 3 μm | A: methanol: acetonitrile 5:95(v/v) B: 2 mM ammonium acetate gradient elution flow rate 0.2 mL/min | [7] |
| Urine | 8-iso-PGF2α | Phenomenex Gemini C 18 110 Å, 50mm × 2.0 mm × 3 μm | A: 0.1% aqueous solution of ammonium hydroxide B: 0.1% ammonium hydroxide in acetonitrile gradient elution | [9] |
| Plasma | 8-iso-15(R)PGF2α 11β-PGF2α 15(R)-PGF2α PGF2α | Synergi Hydro-RP 250 mm × 2.0mm | A: water + 0.01% acetic acid B: methanol gradient elution | [10] |
| Urine | 8-iso-PGF2α 8-iso-15(R)-PGF2α 8-iso-PGF2ß PGF2α | Hypercarb, 5 μm, 1 mm × 150 mm, porous graphitic carbon column | A: water + 0.5% aqueous solution of ammonium hydroxide (pH 9.5) B: acetonitrile:methanol 40:60(v/v) + 0.5% aqueous solution of ammonium hydroxide flow rate 50 μL/min gradient elution | [6] |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Janicka, M.; Kot-Wasik, A.; Kot, J.; Namieśnik, J. Isoprostanes-Biomarkers of Lipid Peroxidation: Their Utility in Evaluating Oxidative Stress and Analysis. Int. J. Mol. Sci. 2010, 11, 4631-4659. https://doi.org/10.3390/ijms11114631
Janicka M, Kot-Wasik A, Kot J, Namieśnik J. Isoprostanes-Biomarkers of Lipid Peroxidation: Their Utility in Evaluating Oxidative Stress and Analysis. International Journal of Molecular Sciences. 2010; 11(11):4631-4659. https://doi.org/10.3390/ijms11114631
Chicago/Turabian StyleJanicka, Monika, Agata Kot-Wasik, Jacek Kot, and Jacek Namieśnik. 2010. "Isoprostanes-Biomarkers of Lipid Peroxidation: Their Utility in Evaluating Oxidative Stress and Analysis" International Journal of Molecular Sciences 11, no. 11: 4631-4659. https://doi.org/10.3390/ijms11114631






