Study of Influencing Factors and the Mechanism of Preparing Triazinedithiol Polymeric Nanofilms on Aluminum Surfaces
Abstract
:1. Introduction
2. Results and Discussion
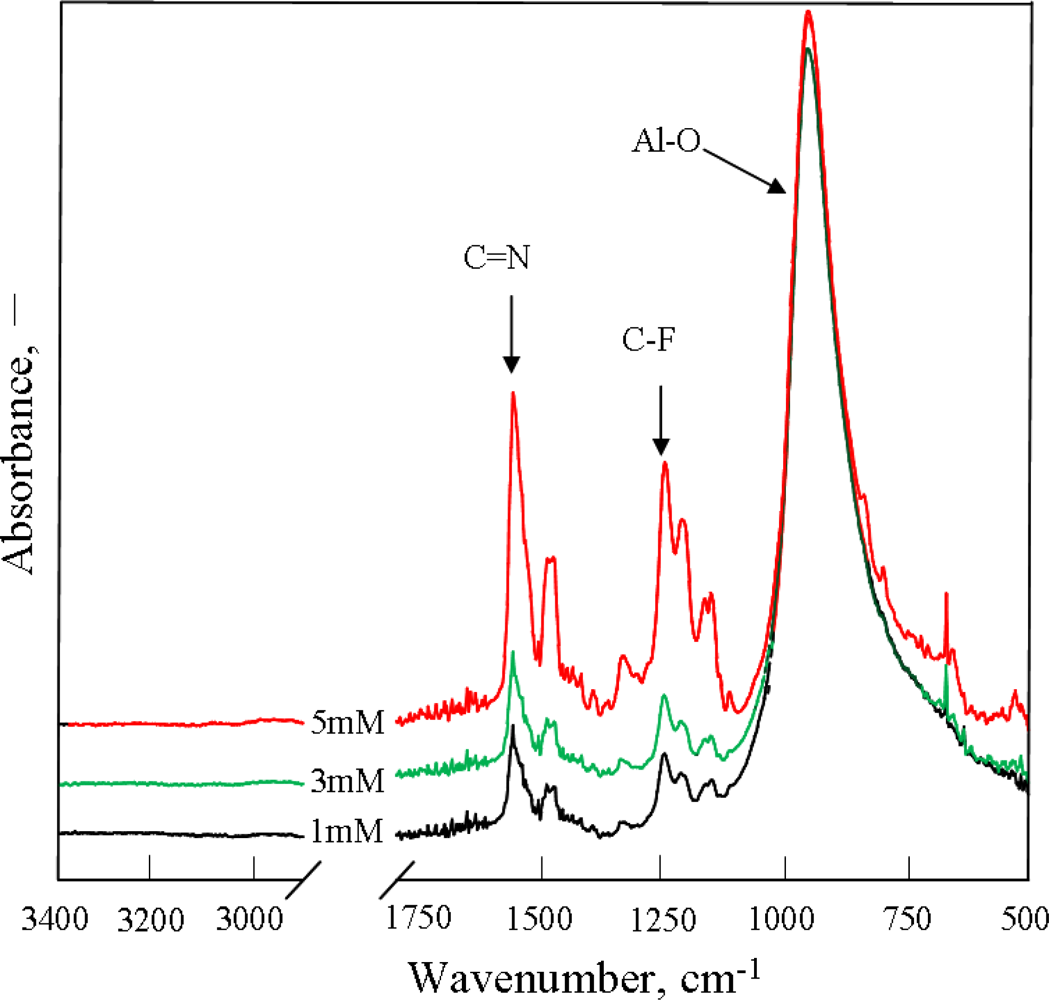
2.1. Effect of Monomer Concentration on PAF17 Nanofilm
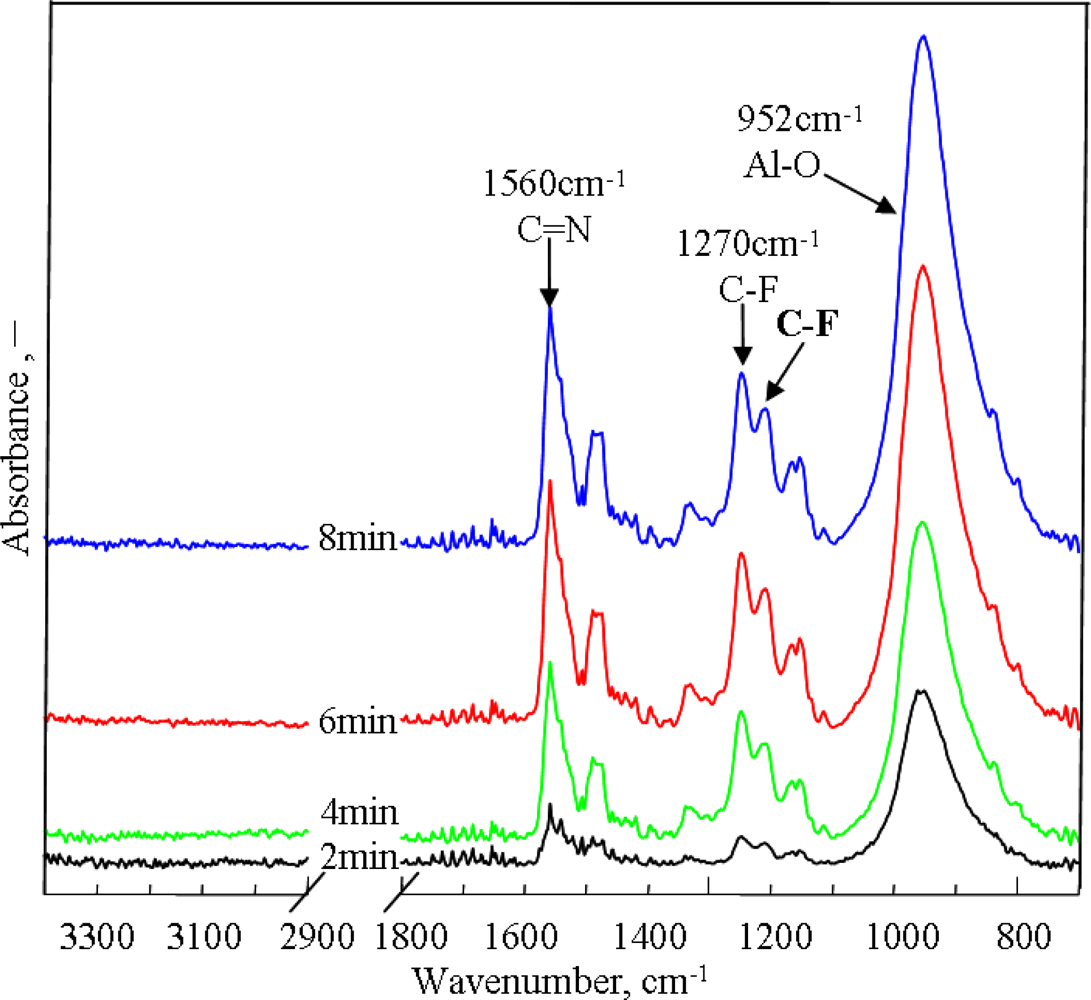
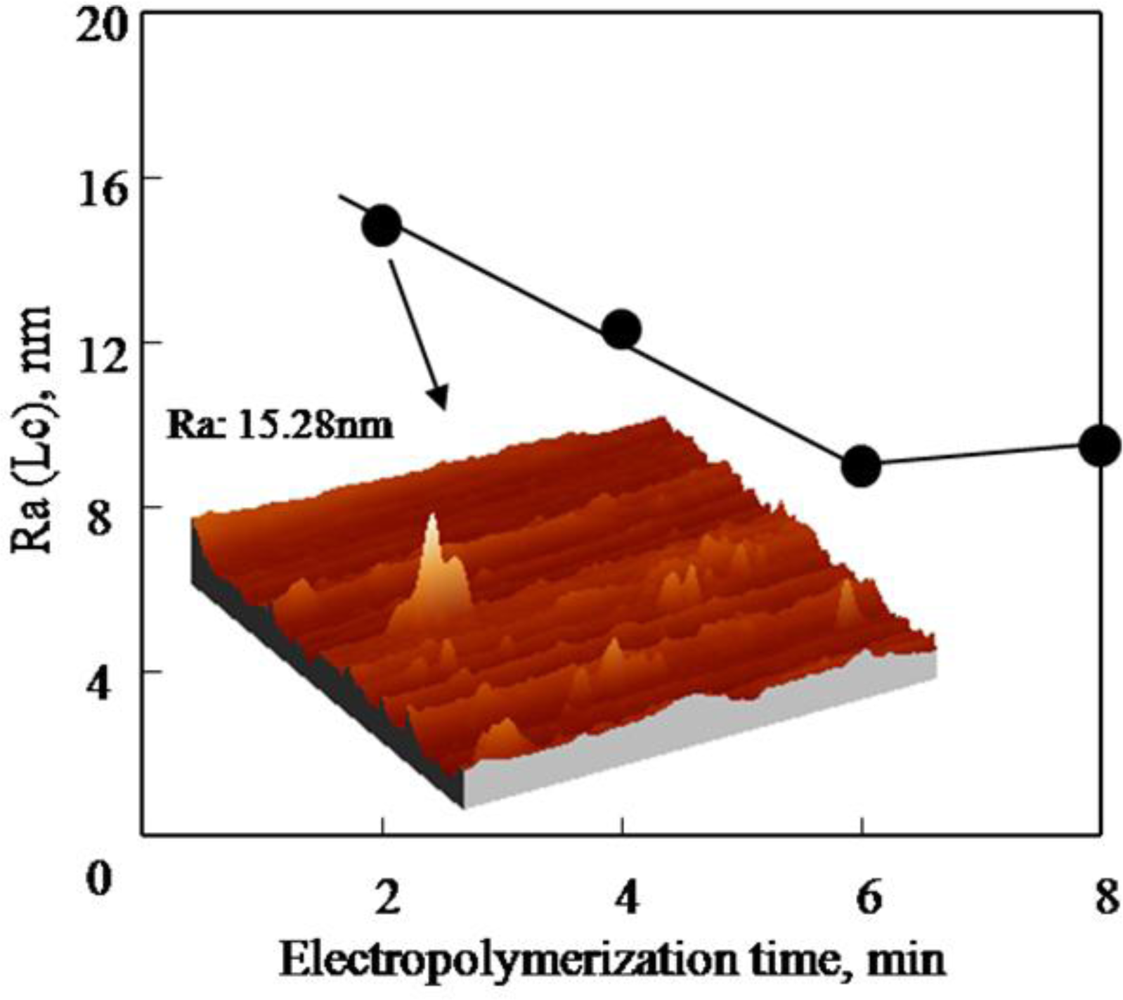
2.2. Effect of Electropolymerization Time on PAF17 Nanofilm
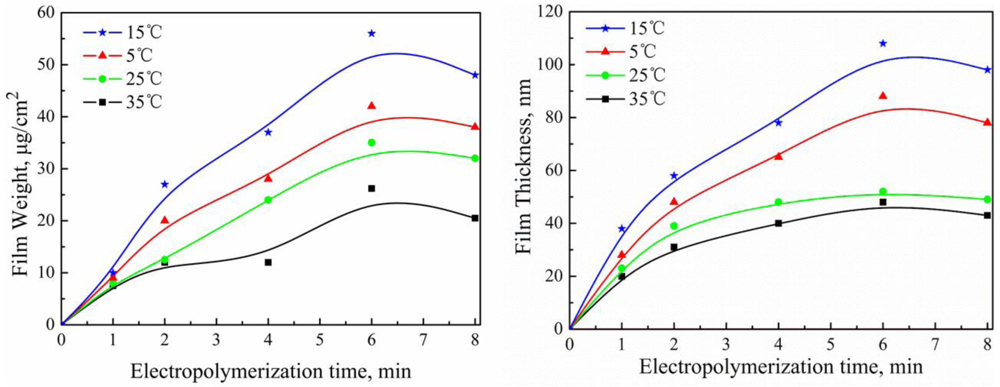
2.3. Effect of Electropolymerization Temperature on PAF17 Nanofilm
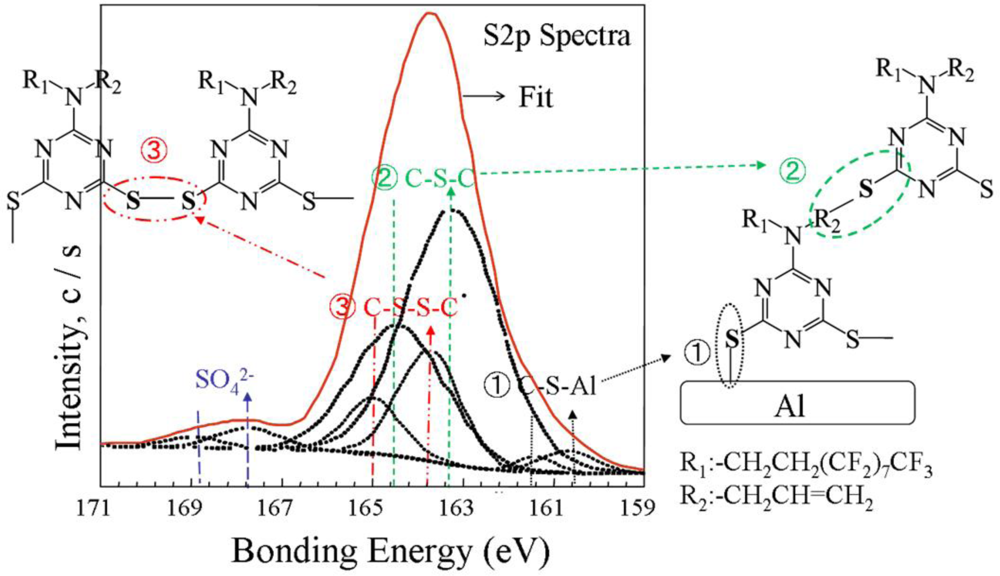
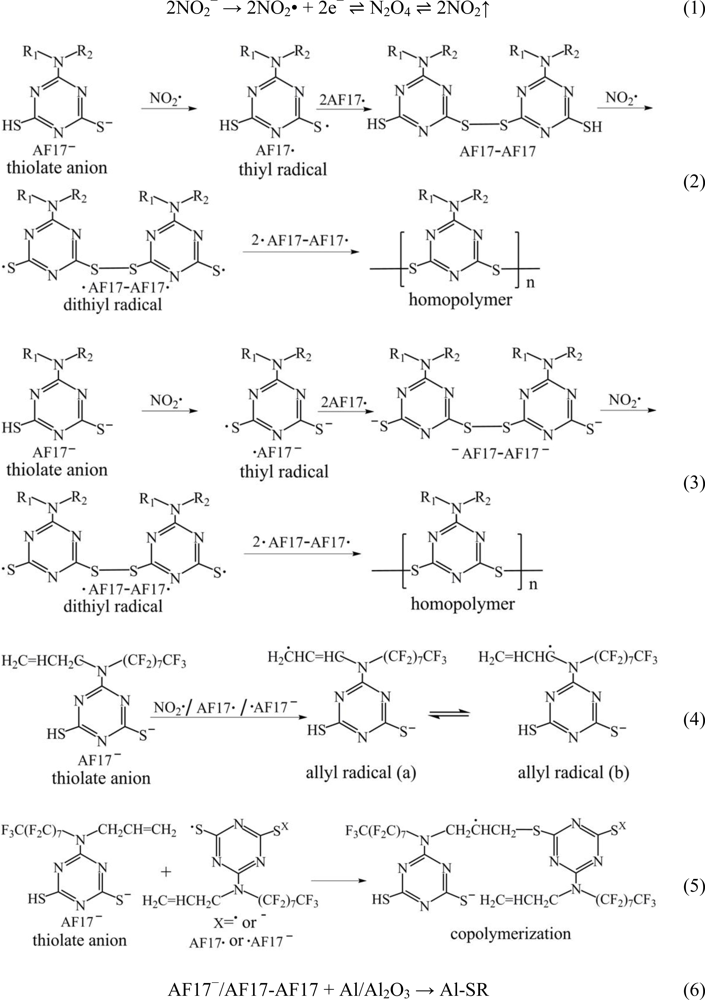
2.4. Electropolymerization Mechanism
3. Experimental Section
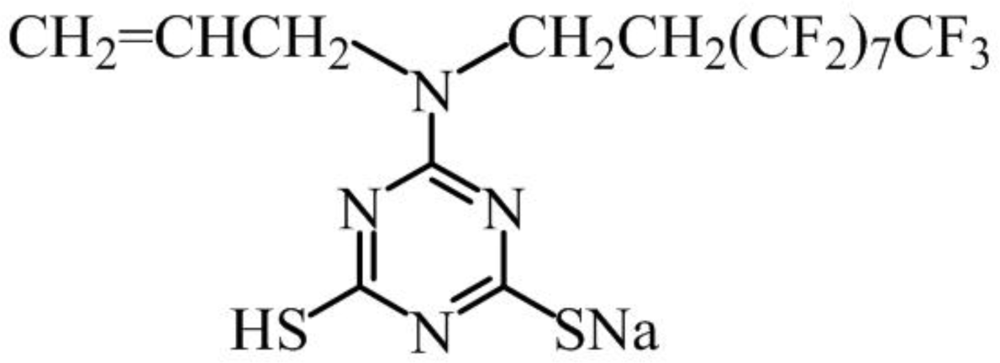
3.1. Experimental Materials
3.2. Preparation of PAF17 Nanofilm under Different Electropolymerization Conditions
3.3. Characterization
4. Conclusions
Acknowledgments
References
- Mori, K; Okai, Y; Horie, H; Yamada, H. The corrosion and inhibition of copper powders. Corros. Sci 1991, 32, 1237–1252. [Google Scholar]
- Baba, H; Kodama, T; Mori, K; Hirahara, H. The corrosion inhibition of copper by potentiostatic anodization in triazinedithiol solutions. Corros. Sci 1997, 39, 555–564. [Google Scholar]
- Kang, Z; Li, Y; Mori, K. Application of polymer plating to inhibit corrosion of magnesium alloy. Mater. Sci. Forum 2005, 488, 661–664. [Google Scholar]
- Mori, K; Asakura, T; Oishi, Y. Electrochemical reaction in the polymer plating of triazine dithiols. Polym. J 2003, 35, 568. [Google Scholar]
- Yamamoto, M; Suzuki, M; Tachikawa, M; Fujishima, A; Miyazaki, T; Hisamitsu, H; Kojima, K; Kadoma, Y. Film Formation from Mixed Solutions of 1,3,5-Triazine-2,4-dithione and Phosphate onto Au, Ag, and Cu Substrates. J. Phys. Chem. C 2008, 112, 6914–6923. [Google Scholar]
- Mori, K; Hirahara, H; Oishi, Y; Katayama, M. Tribological Polymerization of 2-Dibutylamino-1,3,5-Triazine-4,6-Dithiol on Iron Wire during Drawing. Polym. J 1995, 27, 1058–1063. [Google Scholar]
- Mori, K; Sai, S; Miyashita, T; Matsuda, M. Photopolymerization of thin films of triazinedithiols. Langmuir 1991, 7, 1158–1160. [Google Scholar]
- Mori, K; Oishi, Y; Miyashita, T; Matsuda, M. Polymerization of Monomer Films of Triazine Dithiols on a Copper Surface. Polym. Int 1992, 28, 193–199. [Google Scholar]
- Mori, K; Sasaki, Y; Sai, S; Kaneda, S; Hirahara, H; Oishi, Y. Electrochemical polymerization of 2-(dioctylamino)-1, 3, 5-triazine-4, 6-dithiol on iron plates. Langmuir 1995, 11, 1431–1434. [Google Scholar]
- Mori, K; Suzuki, K; Shimizu, K; Oishi, Y. Evaporation polymerization of 6-dibutylamino-1,3,5-triazine-2,4-dithiol on iron plates. Langmuir 2002, 18, 9527–9532. [Google Scholar]
- Wang, F; Mori, K; Kang, Z; Oishi, Y. Magnetic field effects on the polymerization of 6-N,N-dioctylamino-1,3,5-triazine-2,4-dithiol. Heteroatom. Chem 2007, 18, 60–64. [Google Scholar]
- Wang, F; Mori, K; Oishi, Y. Electrochemical polymerization of 6-(N-allyl-1,1,2,2-tetrahydroperfluorodecyl)amino-1,3,5-triazine-2,4-dithiol monosodium on aluminum. Polym. J 2006, 38, 484–489. [Google Scholar]
- Wang, F; Luo, H; Wang, Q; Wang, J; Xu, J. Preparation of superhydrophobic polymeric film on aluminum plates by electrochemical polymerization. Molecules 2009, 14, 4737. [Google Scholar]
- Kang, Z; Ye, Q; Sang, J; Li, Y. Fabrication of super-hydrophobic surface on copper surface by polymer plating. J. Mater. Process. Tech 2009, 209, 4543–4547. [Google Scholar]
- Huang, M; Li, X; Li, S; Zhang, W. Resultful synthesis of polyvinyltetrazole from polyacrylonitrile. React. Funct. Polym 2004, 59, 53–61. [Google Scholar]
- Li, X; Huang, M; Liu, R. Facile synthesis of semi-conducting particles of oxidative melamine/toluidine copolymers with solvatochromism. React. Funct. Polym 2005, 62, 285–294. [Google Scholar]
- Mori, K; Sasaki, Y; Hirahara, H; Oishi, Y. Accelerating effect of NaNO2 on the polymer plating of 6-substituted-1,3,5-triazine-2,4-dithiol mono sodium salts. J. Appl. Polym. Sci 2001, 82, 2300–2309. [Google Scholar]
- Mori, K; Hirahara, H; Oishi, Y; Kumagai, N. Effect of triazine dithiols on the polymer plating of magnesium alloys. Mater Sci Forum 2000. [Google Scholar]

© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, F.; Wang, Y.; Li, Y. Study of Influencing Factors and the Mechanism of Preparing Triazinedithiol Polymeric Nanofilms on Aluminum Surfaces. Int. J. Mol. Sci. 2010, 11, 4715-4725. https://doi.org/10.3390/ijms11114715
Wang F, Wang Y, Li Y. Study of Influencing Factors and the Mechanism of Preparing Triazinedithiol Polymeric Nanofilms on Aluminum Surfaces. International Journal of Molecular Sciences. 2010; 11(11):4715-4725. https://doi.org/10.3390/ijms11114715
Chicago/Turabian StyleWang, Fang, Yabin Wang, and Yanni Li. 2010. "Study of Influencing Factors and the Mechanism of Preparing Triazinedithiol Polymeric Nanofilms on Aluminum Surfaces" International Journal of Molecular Sciences 11, no. 11: 4715-4725. https://doi.org/10.3390/ijms11114715



