Optimization of Total Flavonoid Compound Extraction from Gynura medica Leaf Using Response Surface Methodology and Chemical Composition Analysis
Abstract
:1. Introduction
2. Results and Discussion
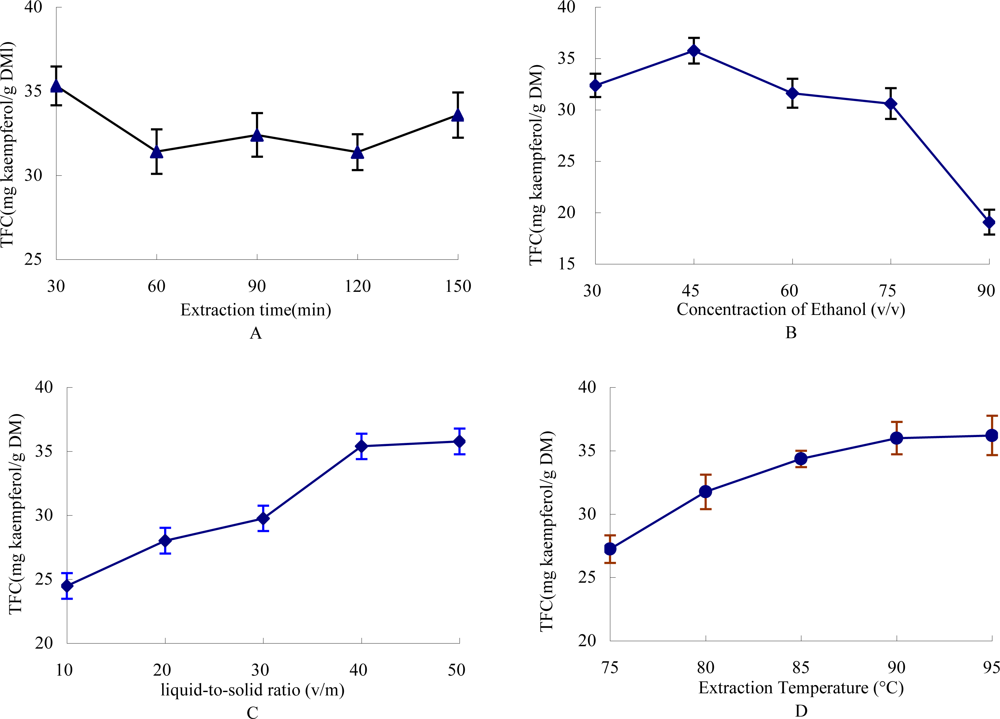
2.1. Effect of Time on the Extraction Yield of TFC
2.2. Effect of Ethanol Concentration on the Yield of TFC
2.3. Effect of Liquid-to-Solid Ratio on the Yield of TFC
2.4. Effect of Extraction Temperature on the Yield of TFC
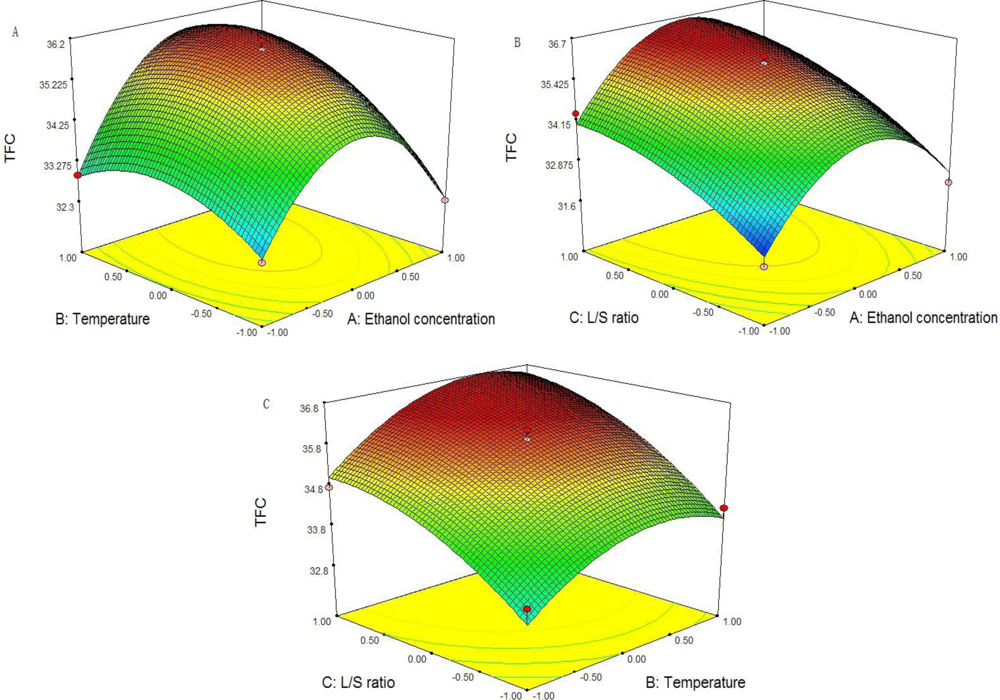
2.5. Optimization of the Yield of TFC
2.6. DPPH and ABTS+ Cation Free Radical Scavenging Capacity
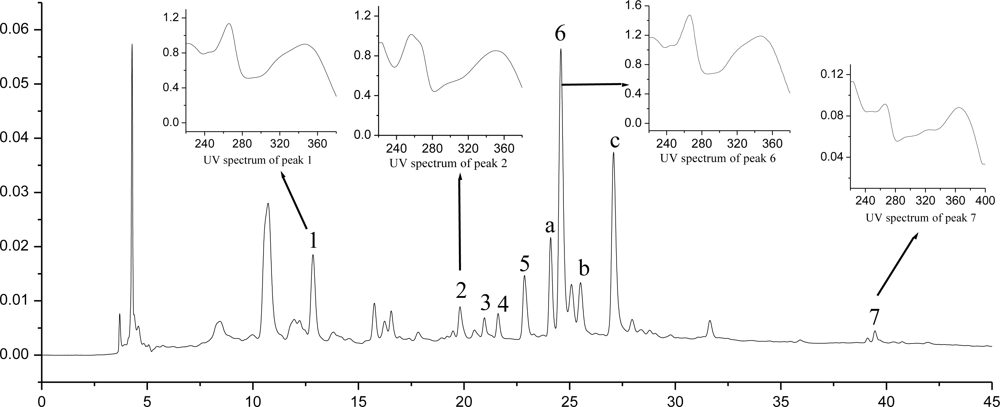
2.7. HPLC-DAD-MS Analysis and Quantification of Flavonoid Compounds
3. Experimental
3.1. Sample Preparation and Chemicals
3.2. Experimental Design
3.4. Solvent Extraction
3.5. Determination of TFC
3.6. DPPH Free Radical Scavenging Activity
3.7. ABTS+ Cation Radical Scavenging Assay
3.8. HPLC-DAD-MS Analyses
3.9. HPLC-DAD Quantification
4. Conclusions
Acknowledgments
References
- Gattuso, G; Barreca, D; Gargiulli, C; Leuzzi, U; Caristi, C. Flavonoid composition of Citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar]
- Gould, KS; Lister, C. Flavonoid functions in plants. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, OM, Markham, KR, Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 397–441. [Google Scholar]
- Heim, KE; Tagliaferro, AR; Bobilya, DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem 2002, 13, 572–584. [Google Scholar]
- Cibin, TR; Devi, DG; Abraham, A. Chemoprevention of skin cancer by the flavonoid fraction of Saraca asoka. Phytother. Res 2010, 24, 666–672. [Google Scholar]
- Fang, S-C; Hsu, C-L; Lin, H-T; Yen, G-C. Anticancer Effects of Flavonoid Derivatives Isolated from Millettia reticulata Benth in SK-Hep-1 Human Hepatocellular Carcinoma Cells. J. Agric. Food Chem 2010, 58, 814–820. [Google Scholar]
- Jin, JH; Lim, H; Kwon, SY; Son, KH; Kim, HP. Anti-inflammatory activity of the total flavonoid fraction from Broussonetia papyrifera in combination with Lonicera japonica. Biomol. Ther 2010, 18, 197–204. [Google Scholar]
- Yam, MF; Lim, V; Salman, IM; Ameer, OZ; Ang, LF; Rosidah, N; Abdulkarim, MF; Abdullah, GZ; Basir, R; Sadikun, A; Asmawi, MZ. HPLC and anti-inflammatory studies of the flavonoid rich chloroform extract fraction of Orthosiphon stamineus leaves. Molecules 2010, 15, 4452–4466. [Google Scholar]
- Jain, A; Singhai, AK; Dixit, VK. A comparative study of ethanol extract of leaves of Tephrosia purpurea pers and the flavonoid isolated for hepatoprotective activity. Indian J. Pharm. Sci 2006, 68, 740–743. [Google Scholar]
- Sannigrahi, S; Mazumder, UK; Pal, DK; Mondal, A; Roy, S. Hepatoprotective potential of flavonoid rich fraction of Enhydra fluctuans against CCl4-induced oxidative damage in rats. Pharmacologyonline 2009, 2, 575–586. [Google Scholar]
- Kouam, J; Etoa, F-X; Mabeku, LB; Fomum, ZT. Sigmoidine L, a new antibacterial flavonoid from Erythrina sigmoidea (Fabaceae). Nat. Prod. Commun 2007, 2, 1105–1108. [Google Scholar]
- Naeem, I; Saddiqe, Z; Patel, A; Hellio, C. Analysis of flavonoid and antimicrobial activity of extracts of Hypericum perforatum. Asian J. Chem 2010, 22, 3596–3600. [Google Scholar]
- Bobe, G; Peterson, JJ; Gridley, G; Hyer, M; Dwyer, JT; Brown, LM. Flavonoid consumption and esophageal cancer among black and white men in the United States. Int. J. Cancer 2009, 125, 1147–1154. [Google Scholar]
- Wang, L; Lee, IM; Zhang, SM; Blumberg, JB; Buring, JE; Sesso, HD. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr 2009, 89, 905–912. [Google Scholar]
- Mao, WH; Han, LJ; Shi, B. Optimization of microwave-assisted extraction of flavonoid from Radix Astragali using response surface methodology. Sep. Sci. Technol 2008, 43, 671–681. [Google Scholar]
- Yang, YK; Wu, JK. A newly plant of Gynura genus. Chin. Acad. Med. Mag. Org 2004, 4, 555–557. [Google Scholar]
- Jin, JS; Kang, FX; Ni, ZD; Fang, J. Effects of aquatic extract of Gynura Medica on anti-hyperglycemia and improving insulin secretion. Anhui Med. Pharm. J 2009, 13, 256–258. [Google Scholar]
- Dong, MZ; Shu, WW. Study on hypoglycemic effects of gynura medica extraction. Strait Pharm. J 2008, 20, 25–27. [Google Scholar]
- Chen, L; Li, H-Q; Song, H-T; Zhang, G-G. A new cerebroside from Gynura divaricata. Fitoterapia 2009, 80, 517–520. [Google Scholar]
- Hou, W-C; Lin, R-D; Lee, T-H; Huang, Y-H; Hsu, F-L; Lee, M-H. The phenolic constituents and free radical scavenging activities of Gynura formosana Kiamnra. J. Sci. Food Agric 2005, 85, 615–621. [Google Scholar]
- Lin, W-Y; Yen, M-H; Teng, C-M; Tsai, I-L; Chen, I-S. Cerebrosides from the rhizomes of Gynura japonica. J. Chin. Chem. Soc. (Taipei, Taiwan) 2004, 51, 1429–1434. [Google Scholar]
- Rana, VS; Blazquez, MA. Chemical constituents of Gynura cusimbua aerial parts. J. Essent. Oil Res 2007, 19, 21–22. [Google Scholar]
- Ginjom, IR; D'Arcy, BR; Caffin, NA; Gidley, MJ. Phenolic Contents and Antioxidant Activities of Major Australian Red Wines throughout the Winemaking Process. J. Agric. Food Chem 2010, 58, 10133–10142. [Google Scholar]
- Shi, F; Jia, X; Zhao, C; Chen, Y. Antioxidant activities of various extracts from Artemisisa selengensis Turcz (LuHao). Molecules 2010, 15, 4934–4946. [Google Scholar]
- Le Gall, G; DuPont, MS; Mellon, FA; Davis, AL; Collins, GJ; Verhoeyen, ME; Colquhoun, IJ. Characterization and content of flavonoid glycosides in genetically modified tomato (Lycopersicon esculentum) fruits. J. Agric. Food Chem 2003, 51, 2438–2446. [Google Scholar]
- Lin, LZ; Harnly, JN. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.). J. Agric. Food Chem 2008, 56, 9094–9101. [Google Scholar]
- Chen, L; Song, Z; Wang, J; Song, H; Wang, J; Zhang, G; Qin, L. Chemical constituents from aerial parts of Gynura divaricata. Zhongcaoyao 2010, 41, 373–375. [Google Scholar]
- Yam, MF; Sadikun, A; Asmawi, MZ. Antioxidant Potential of Gynura procumbens. Pharm. Biol 2008, 46, 616–625. [Google Scholar]
- Pietta, P; Gardana, C; Mauri, P; Zecca, L. High-performance liquid chromatographic analysis of flavonol glycosides of Solidago virgaurea. J. Chromatogr 1991, 558, 296–301. [Google Scholar]
- Apati, P; Houghton, PJ; Kery, A. HPLC investigation of antioxidant components in Solidaginis herba. Acta Pharm. Hung 2004, 74, 223–231. [Google Scholar]
- Lin, LZ; Harnly, JM. A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J. Agric. Food Chem 2007, 55, 1084–1096. [Google Scholar]
- Akowuah, GA; Sadikun, A; Mariam, A. Flavonoid identification and hypoglycemic studies of the butanol fraction from Gynura procumbens. Pharm. Biol 2002, 40, 405–410. [Google Scholar]
- Hu, Y; Li, W-L; Lin, H-W; Zhou, M; Ren, B-R. Method for separation and identification of chemical constituents of Gynura divaricata. Zhongguo Tianran Yaowu 2006, 4, 156–158. [Google Scholar]
- Lu, H; Pei, Y; Li, W. Studies on flavonoids from Gynura bicolor DC. Zhongguo Xiandai Yingyong Yaoxue 2010, 27, 613–614. [Google Scholar]
- Lin, LZ; Harnly, JM. Identification of Hydroxycinnamoylquinic Acids of Arnica Flowers and Burdock Roots Using a Standardized LC-DAD-ESI/MS Profiling Method. J. Agric. Food Chem 2008, 56, 10105–10114. [Google Scholar]
- Gan, CY; Latiff, AA. Optimization of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem 2011, 124, 1277–1283. [Google Scholar]
- Basniwal, PK; Suthar, M; Rathore, GS; Gupta, R; Kumar, V; Pareek, A; Jain, A. In-vitro antioxidant activity of hot aqueous extract of Helicteres isora Linn. fruits. Nat. Prod. Radiance 2009, 8, 483–487. [Google Scholar]
- Amarowicz, R; Estrella, I; Hernandez, T; Duenas, M; Troszynska, A; Kosinska, A; Pegg, RB. Antioxidant activity of a red lentil extract and its fractions. Int. J. Mol. Sci 2009, 10, 5513–5527. [Google Scholar]
| Variables | Coded Levels of Variables | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Ethanol concentration (X1) | 30 | 45 | 60 |
| Temperature (X2) | 85 | 90 | 95 |
| liquid-to-solid ratio (X3) | 30 | 40 | 50 |
| Test Run No. | Coded Levels of Variables | TFC mg kaempferol/g DM | ||
|---|---|---|---|---|
| X1 | X2 | X3 | ||
| 1 | 1 | −1 | 0 | 32.46 |
| 2 | −1 | 1 | 0 | 32.94 |
| 3 | 0 | −1 | 1 | 34.75 |
| 4 | 0 | 1 | 1 | 36.22 |
| 5 | 0 | −1 | −1 | 33.36 |
| 6 | 1 | 1 | 0 | 34.68 |
| 7 | 1 | 0 | −1 | 32.18 |
| 8 | −1 | 0 | 1 | 34.39 |
| 9 | −1 | 0 | −1 | 31.66 |
| 10 | 1 | −1 | 0 | 32.32 |
| 11 | 0 | 1 | −1 | 34.24 |
| 12 | 1 | 0 | 1 | 35.29 |
| 13 | 0 | 0 | 0 | 35.86 |
| 14 | 0 | 0 | 0 | 35.91 |
| 15 | 0 | 0 | 0 | 36.13 |
| SD | DF | SS | F value | Prob > F | S |
|---|---|---|---|---|---|
| Model | 9 | 33.71 | 22.48 | 0.0016 | ** |
| X1 | 1 | 1.13 | 6.79 | 0.0480 | * |
| X2 | 1 | 3.36 | 20.16 | 0.0065 | ** |
| X3 | 1 | 10.58 | 63.47 | 0.0005 | ** |
| X1X2 | 1 | 0.88 | 5.27 | 0.0702 | |
| X1X3 | 1 | 0.037 | 0.22 | 0.6587 | |
| X2X3 | 1 | 0.086 | 0.52 | 0.5044 | |
| X12 | 1 | 15.74 | 94.48 | 0.0002 | ** |
| X22 | 1 | 2.37 | 14.22 | 0.0130 | * |
| X32 | 1 | 1.00 | 6.00 | 0.0580 | |
| Lack of fit | 3 | 0.79 | 12.42 | 0.0754 |
| Final Concentration (μg/mL DM) | DPPH | ||
|---|---|---|---|
| RSA% | μmol Q/g DM | μmol T/g DM | |
| 40 | 9.77 ± 1.29 | 50.92 ± 5.35 | 137.30 ± 8.98 |
| 80 | 15.93 ± 0.80 | 38.20 ± 1.65 | 90.03 ± 2.78 |
| 120 | 32.50 ± 1.20 | 47.83 ± 1.64 | 97.38 ± 2.74 |
| 160 | 47.41 ± 0.67 | 51.65 ± 0.69 | 99.65 ± 1.16 |
| 200 | 63.69 ± 1.66 | 54.78 ± 1.37 | 102.31 ± 2.30 |
| Final Concentration (μg/mL DM) | ABTS+ | ||
|---|---|---|---|
| RSA% | μmol Q/g DM | μmol T/g DM | |
| 20 | 27.35 ± 14.0 | 74.98 ± 4.79 | 192.81 ± 10.25 |
| 30 | 39.70 ± 1.23 | 87.91 ± 3.78 | 209.68 ± 8.09 |
| 40 | 52.95 ± 0.65 | 94.76 ± 1.49 | 220.74 ± 3.19 |
| 50 | 66.41 ± 0.86 | 101.98 ± 1.59 | 231.15 ± 3.40 |
| 60 | 81.53 ± 1.31 | 108.21 ± 2.01 | 242.31 ± 4.30 |
| Peak No. | RT (min) | λmax (nm) | Product Ions (ESI−, m/z) | Product Ions (ESI+, m/z) | Identification Compounds | Content mg/g DM |
|---|---|---|---|---|---|---|
| 1 | 12.85 | 264, 347 | 609 | 611, 449, 287 | kaempferol-3,7-di-O-β-d-glucoside | 0.155 |
| 2 | 19.81 | 254, 356 | 609 | 611, 465, 303 | quercetin-3-O-rutinoside | 0.053 |
| 3 | 20.96 | 256, 354 | 463 | 465, 303 | quercetin-3-O-β-d-glucoside | 0.038 |
| 4 | 21.61 | 265, 346 | 593 | 595, 449, 287 | kaempferol-3-O-robinobioside | 0.040 |
| 5 | 22.86 | 265, 347 | 593 | 595, 449, 287 | kaempferol-3-O-rutinoside | 0.102 |
| 6 | 24.59 | 265, 347 | 447 | 449, 287 | kaempferol-3-O-β-d-glucoside | 0.423 |
| 7 | 39.46 | 266, 367 | 285 | 287 | kaempferol | 0.018 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, W.; Yu, Y.; Yang, R.; Wan, C.; Xu, B.; Cao, S. Optimization of Total Flavonoid Compound Extraction from Gynura medica Leaf Using Response Surface Methodology and Chemical Composition Analysis. Int. J. Mol. Sci. 2010, 11, 4750-4763. https://doi.org/10.3390/ijms11114750
Liu W, Yu Y, Yang R, Wan C, Xu B, Cao S. Optimization of Total Flavonoid Compound Extraction from Gynura medica Leaf Using Response Surface Methodology and Chemical Composition Analysis. International Journal of Molecular Sciences. 2010; 11(11):4750-4763. https://doi.org/10.3390/ijms11114750
Chicago/Turabian StyleLiu, Wei, Yanying Yu, Ruzhen Yang, Chunpeng Wan, Binbin Xu, and Shuwen Cao. 2010. "Optimization of Total Flavonoid Compound Extraction from Gynura medica Leaf Using Response Surface Methodology and Chemical Composition Analysis" International Journal of Molecular Sciences 11, no. 11: 4750-4763. https://doi.org/10.3390/ijms11114750






