Optimization of Bacterial Plasmid Transformation Using Nanomaterials Based on the Yoshida Effect
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Parameters for Plasmid Transformation by Means of Sepiolite
2.2. Plasmid Transformation by Means of Sepiolite and Vortex Operation
2.3. Plasmid Transformation by Means of CNTs
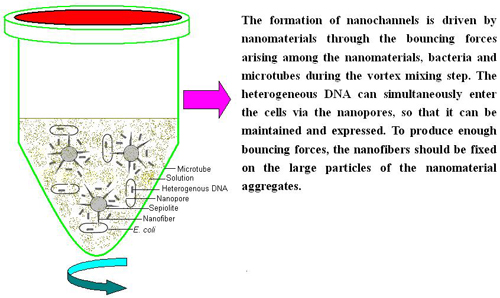
2.4. Mechanism for the Plasmid Transformation by Means of Nanomaterials
3. Experimental Section
3.1. Materials and Strains
3.2. Cell Culture
3.3. Optimization of Parameters for Plasmid Transformation by Means of Sepiolite
3.4. Plasmid Transformation by Means of Sepiolite and Vortex Operation
3.5. Plasmid Transformation by Means of CNTs
3.6. Plasmid Transformation by the Calcium Chloride Method
3.7. Mechanism for the Plasmid Transformation by Means of Nanomaterials
4. Conclusions
Acknowledgments
References
- Yoshida, N; Ikeda, T; Yoshida, T. Chrysotile asbestos fibers mediate transformation of Escherichia coli by exogenous plasmid DNA. FEMS Microbiol. Lett 2001, 195, 133–137. [Google Scholar]
- Landrigan, PJ; Nicholson, WJ; Suzuki, Y. The hazards of chrysotile asbestos: a critical review. Ind. Health 1999, 37, 271–280. [Google Scholar]
- Yoshida, N; Ide, K. Plasmid DNA is released from nanosized acicular material surface by low molecular weight oligonucleotides: exogenous plasmid acquisition mechanism for penetration intermediates based on the Yoshida effect. Appl. Microbiol. Biotechnol 2008, 80, 813–821. [Google Scholar]
- Wilharm, G; Lepka, D; Faber, F. A simple and rapid method of bacterial transformation. J. Microbiol. Method 2010, 80, 215–216. [Google Scholar]
- Yoshida, N; Sato, M. Plasmid uptake by bacteria: a comparison of methods and efficiencies. Appl. Microbiol. Biotechnol 2009, 83, 791–798. [Google Scholar]
- Zhang, Z; Shen, C; Wang, M; Han, H; Cao, X. Aqueous suspension of carbon nanotubes enhances the specificity of long PCR. Biotechniques 2008, 44, 537–545. [Google Scholar]
- Rojas-Chapana, J; Troszczynska, J; Firkowska, I. Multi-walled carbon nanotubes for plasmid delivery into Escherichia coli cells. Lab. Chip 2005, 5, 536–539. [Google Scholar]
- Sung, K; Khan, SA; Nawaz, MS. A simple and efficient Triton X-100 boiling and chloroform extraction method of RNA isolation from Gram-positive and Gram-negative bacteria. FEMS Microbiol. Lett 2003, 229, 97–101. [Google Scholar]
- Joshi, RP; Schoenbach, KH. Mechanism for membrane electroporation irreversibility under high-intensity, ultrashort electrical pulse conditions. Phys. Rev. E 2002, 66, 052901. [Google Scholar]
- Yoshida, N. Discovery and application of the Yoshida effect: Nano-sized acicular materials enable penetration of bacterial cells by sliding friction force. Rec. Pat. Biotechnol 2007, 1, 194–201. [Google Scholar]
- Yoshida, N; Saeki, Y. Chestnut bur-shaped aggregates of chrysotile particles enable inoculation of Escherichia coli cells with plasmid DNA. Appl. Microbiol. Biotechnol 2004, 65, 566–575. [Google Scholar]
- Yoshida, N; Kodama, K; Nakata, K. Escherichia coli cells penetrated by chrysotile fibers are transformed to antibiotic resistance by incorporation of exogenous plasmid DNA. Appl. Microbiol. Biotechnol 2002, 60, 461–468. [Google Scholar]
- Yoshida, N; Nakajima-Kambe, T; Matsuki, K. Novel plasmid transformation method mediated by chrysotile, sliding friction, and elastic body exposure. Anal. Chem. Insights 2007, 2, 9–15. [Google Scholar]
- Tu, Z; He, G; Li, K. An improved system for competent cell preparation and high efficiency plasmid transformation using different Escherichia coli strains. Electron. J. Biotechnol 2005, 8, 113–120. [Google Scholar]





© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tan, H.; Fu, L.; Seno, M. Optimization of Bacterial Plasmid Transformation Using Nanomaterials Based on the Yoshida Effect. Int. J. Mol. Sci. 2010, 11, 4962-4972. https://doi.org/10.3390/ijms11124962
Tan H, Fu L, Seno M. Optimization of Bacterial Plasmid Transformation Using Nanomaterials Based on the Yoshida Effect. International Journal of Molecular Sciences. 2010; 11(12):4962-4972. https://doi.org/10.3390/ijms11124962
Chicago/Turabian StyleTan, Haidong, Li Fu, and Masaharu Seno. 2010. "Optimization of Bacterial Plasmid Transformation Using Nanomaterials Based on the Yoshida Effect" International Journal of Molecular Sciences 11, no. 12: 4962-4972. https://doi.org/10.3390/ijms11124962