Liquid-Liquid Phase Transition and Glass Transition in a Monoatomic Model System
Abstract
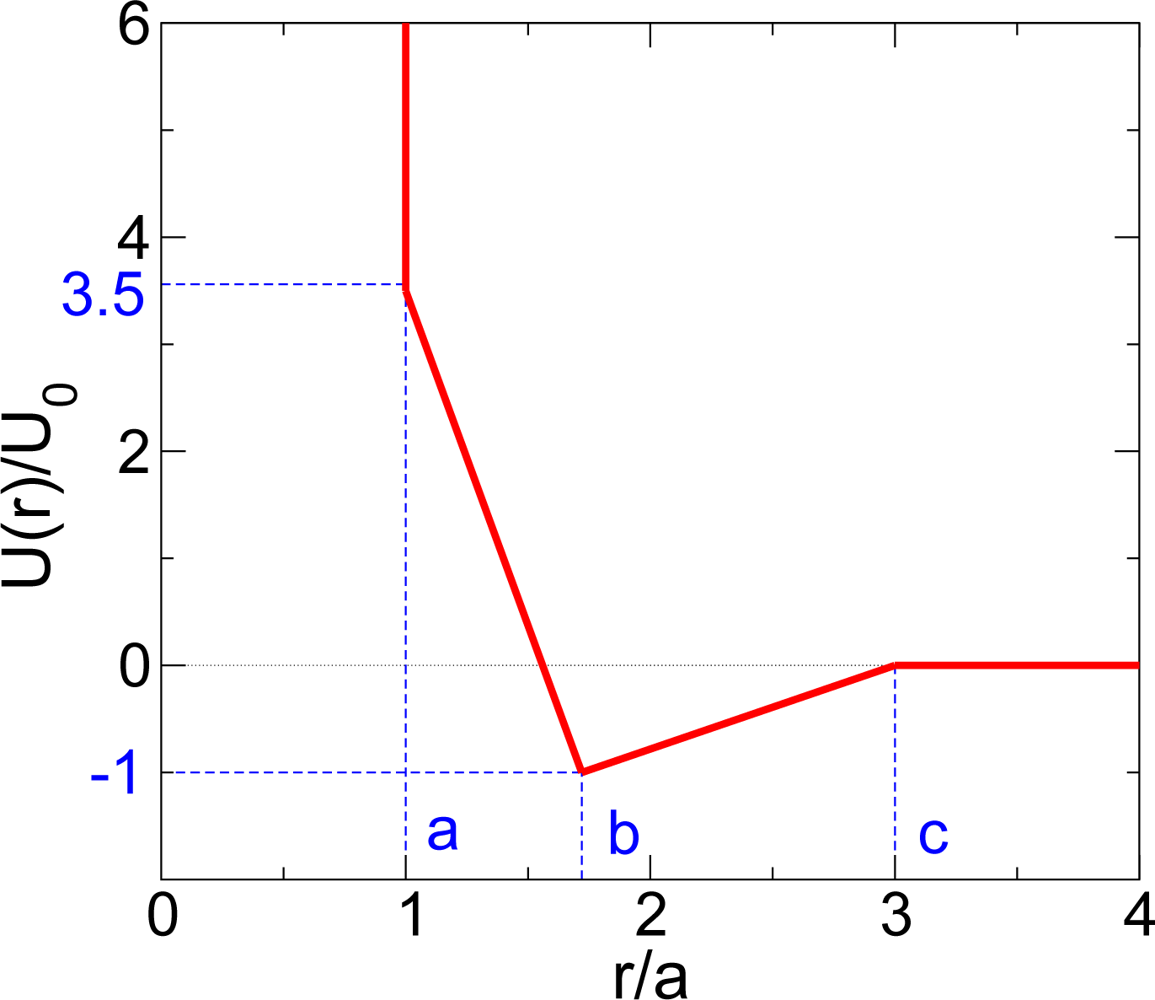
:1. Introduction
2. Polyamorphism in the Liquid States
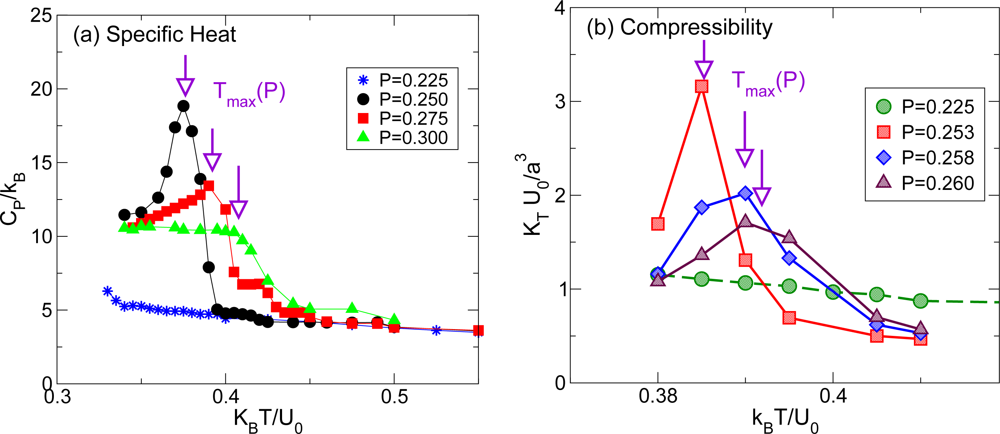
2.1. Liquid-Liquid Phase Transition
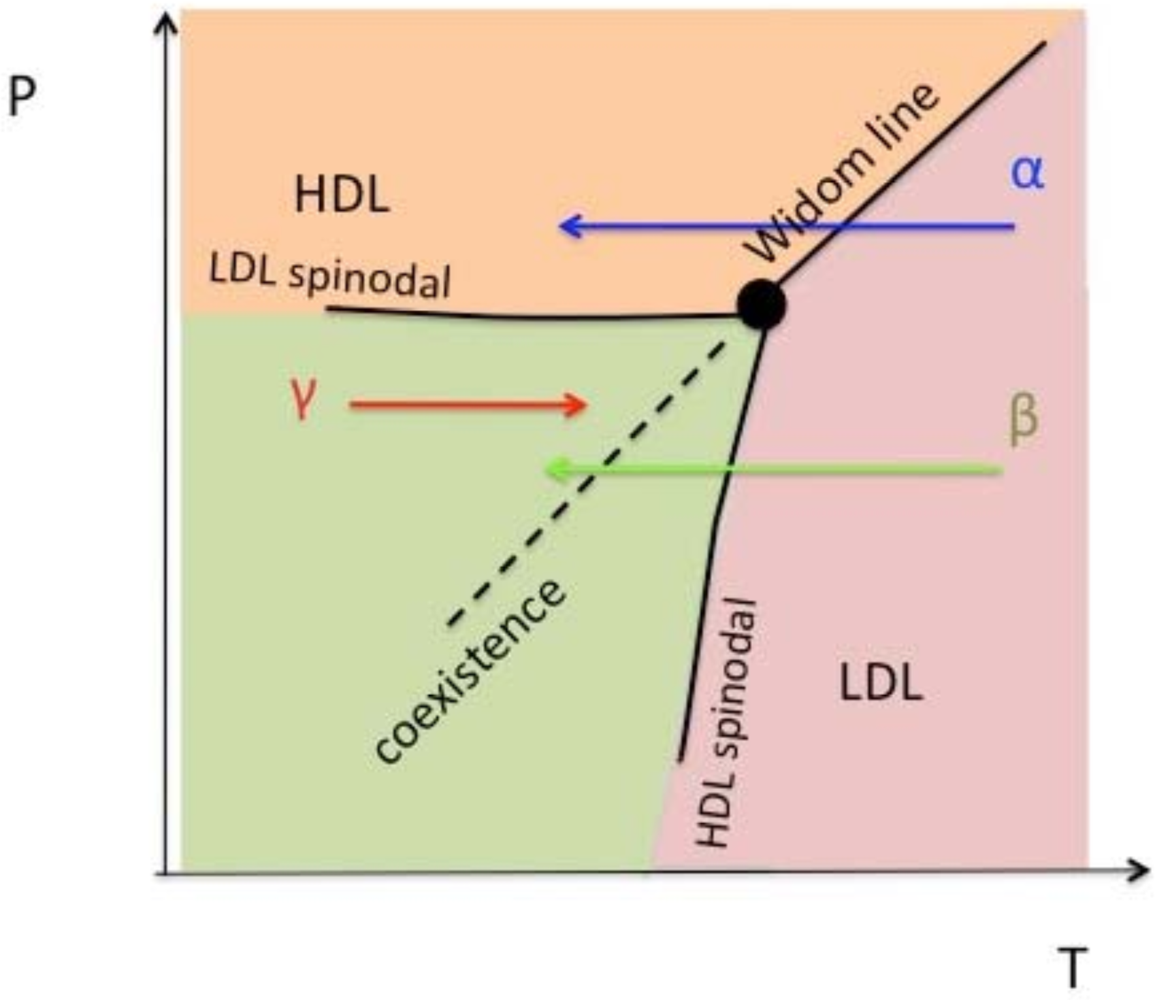
2.2. Liquid-Liquid Transition and the Widom Line
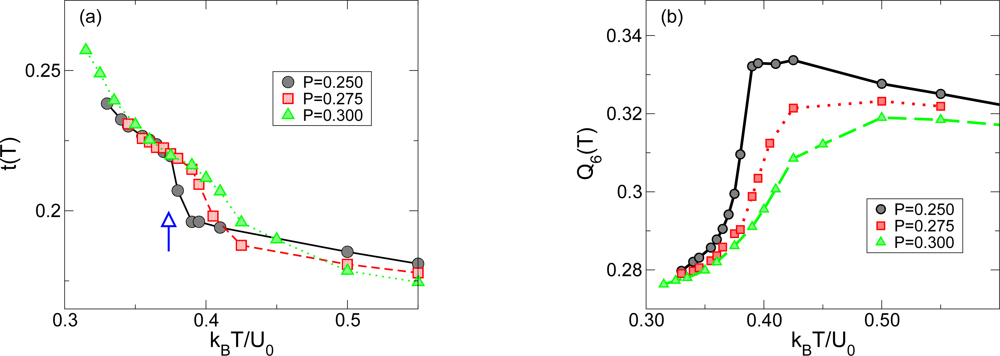
2.3. Structural Changes and Liquid-Liquid Phase Transition
2.4. Dynamics Crossover and Liquid-Liquid Phase Transition
3. Liquid—Glass Transformations
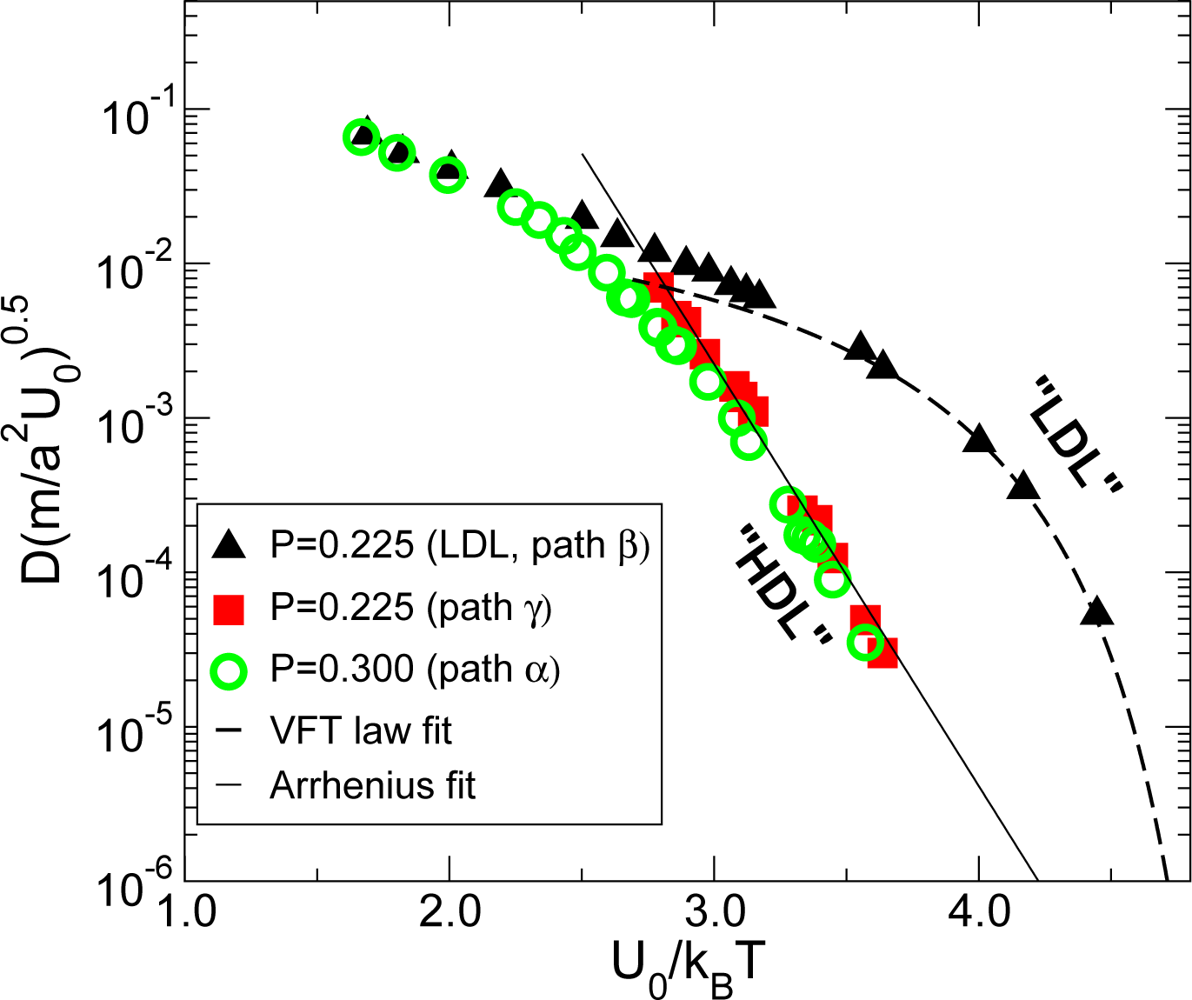
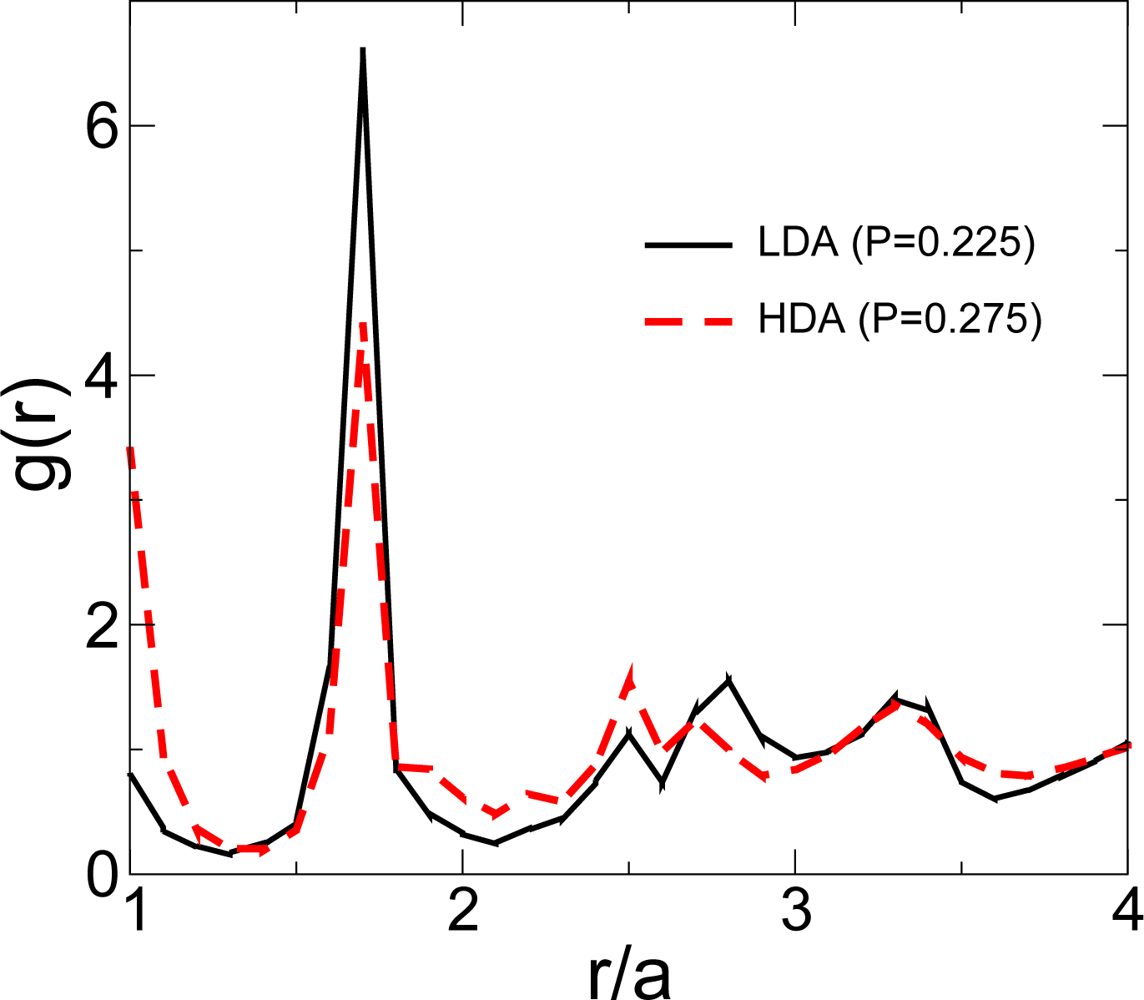
3.1. Low Density Amorphous and High Density Amorphous
3.2. Pressure Dependence of the Glass Transition
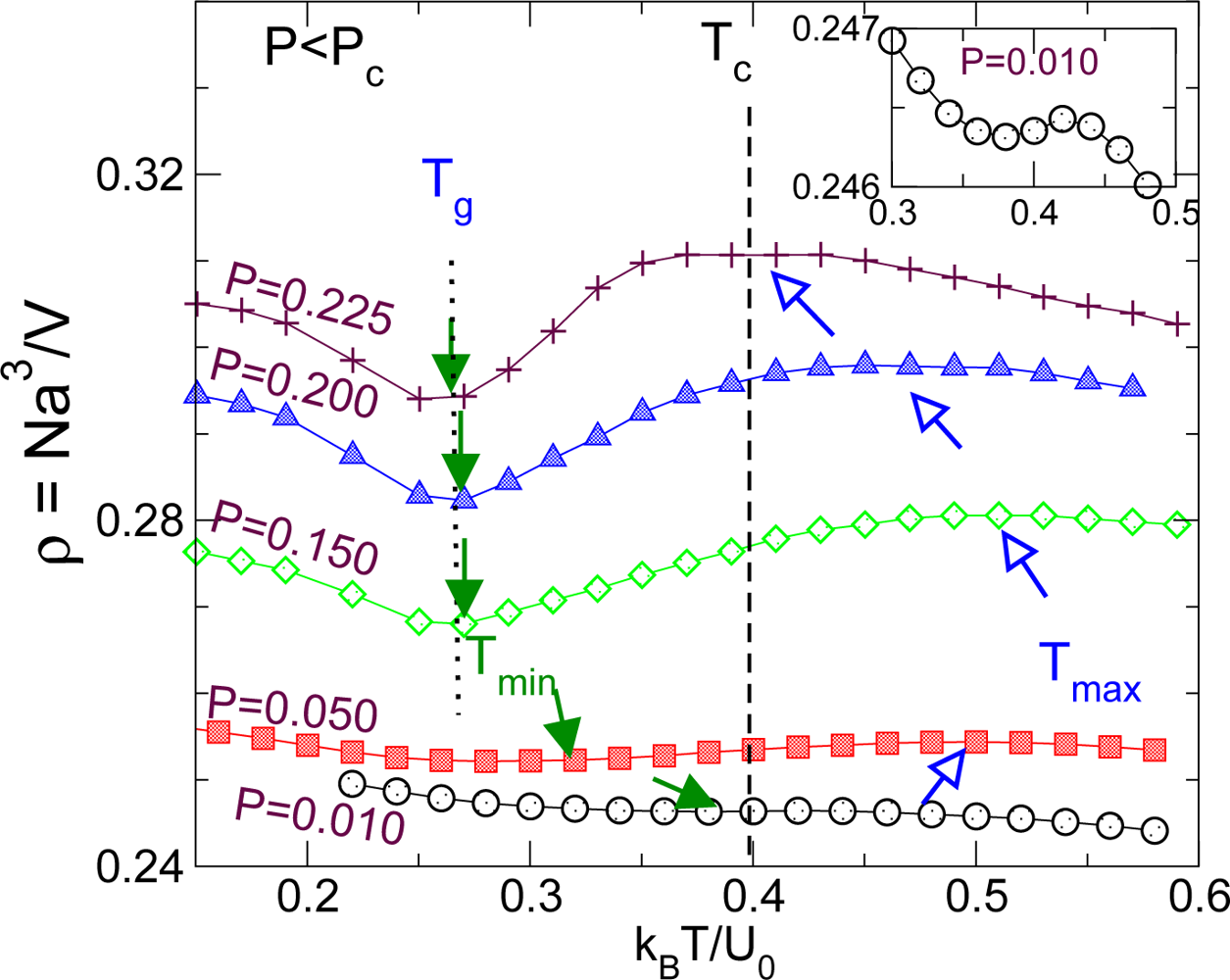
3.3. Density Minimum and Glass Transition
4. Conclusions
Acknowledgments
References
- Wilding, MC; Wilson, M; Mcmillan, PF. Structural studies and polymorphism in amorphous solids and liquids at high pressure. Chem. Soc. Rev 2006, 35, 964–986. [Google Scholar]
- McMillan, PF. Polyamorphic transformations in liquids and glasses. J. Mat. Chem 2004, 14, 1506–1512. [Google Scholar]
- Angell, CA. Insights into phases of liquid water from study of its unusual glass-forming properties. Science 2008, 319, 582–587. [Google Scholar]
- Debenedetti, PG. Supercooled and glassy water. J. Phys. Condens. Mat 2003, 15, R1669–R1726. [Google Scholar]
- Angell, CA. Amorphous water. Ann. Rev. Phys. Chem 2004, 55, 559–583. [Google Scholar]
- Debenedetti, PG; Stanley, HE. Supercooled and glassy water. Phys. Today 2003, 56, 40–46. [Google Scholar]
- Mishima, O; Stanley, HE. The relationship between liquid, supercooled and glassy water. Nature 1998, 396, 329–335. [Google Scholar]
- Mishima, O; Calvert, LD; Whalley, E. An apparent first-order transition between two amorphous phases of ice induced by pressure. Nature 1985, 314, 76–78. [Google Scholar]
- Mishima, O; Calvert, LD; Whalley, E. Melting ice-I at 77K and 10kbar–A new method of making amorphous solids. Nature 1984, 310, 393–395. [Google Scholar]
- Mishima, O. Relationship between melting and amorphization of ice. Nature 1996, 384, 546–549. [Google Scholar]
- Loerting, T; Salzmann, C; Kohl, I; Mayer, E; Hallbrucker, A. A second distinct structural “state” of high-density amorphous ice at 77 K and 1 bar. Phys. Chem. Chem. Phys 2001, 3, 5355–5357. [Google Scholar]
- Finney, JL; Bowron, DT; Soper, AK; Loerting, T; Mayer, E; Hallbrucker, A. Structure of a new dense amorphous ice. Phys. Rev. Lett 2002, 89, 205503. [Google Scholar]
- New Kinds of Phase Transitions: Transformations in Disordered Substances; Brazhkin, V; Buldyrev, SV; Ryzhov, VN; Stanley, HE (Eds.) NATO Advanced Research Workshop: Brussels Belgium, 2002.
- Loerting, T; Brazhkin, VV; Morishita, T. Multiple amorphous-amorphous transitions. Adv. Chem. Phys 2009, 143, 29–82. [Google Scholar]
- Katayama, Y; Mizutani, T; Tsumi, K; Shinomura, O; Yamakata, M. A first-order liquid-liquid phase transition in phosphorus. Nature 2000, 403, 170–173. [Google Scholar]
- Monaco, G; Falconi, S; Crichton, WA; Mezouar, M. Nature of the first-order phase transition in fluid phosphorus at high temperature and pressure. Phys. Rev. Lett 2003, 90, 255701. [Google Scholar]
- Bhat, H; Molinero, V; Solomon, V; Soignard, E; Sastry, S; Yarger, JL; Angell, CA. Vitrification of a monatomic metallic liquid. Nature 2007, 448, 787–790. [Google Scholar]
- Kurita, R; Tanaka, H. Critical-like phenomena associated with liquid-liquid transition in a molecular liquid. Science 2004, 306, 845–848. [Google Scholar]
- Sheng, HW; Liu, HZ; Cheng, YQ; Wen, J; Lee, PL; Luo, WK; Shastri, SD; Ma, E. Polyamorphism in a metallic glass. Nat. Mater 2007, 6, 192–197. [Google Scholar]
- Sen, S; Gaudio, S; Aitken, BG; Lesher, CE. Observation of a pressure-induced first-order polyamorphic transition in a chalcogenide glass at ambient temperature. Phys. Rev. Lett 2006, 97, 025504. [Google Scholar]
- Poole, PH; Sciortino, F; Essmann, U; Stanley, HE. Phase-behavior of metastable water. Nature 1992, 360, 324–328. [Google Scholar]
- Zanotti, J-M; Bellissent-Funel, M-C; Chen, S-H. Experimental evidence of a liquid-liquid transition in interfacial water. Europhys. Lett 2005, 71, 91–97. [Google Scholar]
- Chen, S-H; Mallamace, F; Liu, L; Liu, DZ; Chu, XQ; Zhang, Y; Kim, C; Faraone, A; Mou, C-Y; Fratini, E; Baglioni, P; Kolesnikov, AI; Garcia-Sakai, V. Dynamic crossover phenomenon in confined supercooled water and its relation to the existence of a liquid-liquid critical point in water. Proceedings of 5th International Workshop on Complex Systems, Sendai, Japan, 25–28 September 2007.
- Liu, L; Chen, S-H; Faraone, A; Yen, CW; Mou, CY. Pressure dependence of fragile-to-strong transition and a possible second critical point in supercooled confined water. Phys. Rev. Lett 2005, 95, 117802. [Google Scholar]
- Chen, S-H; Mallamace, F; Mou, CY; Broccio, M; Corsaro, C; Faraone, A; Liu, L. The violation of the Stokes-Einstein relation in supercooled water. Proc. Nat. Acad. Sci. USA 2006, 103, 12974–12978. [Google Scholar]
- Mallamace, F; Broccio, M; Corsaro, C; Faraone, A; Majolino, D; Venuti, V; Liu, L; Mou, CY; Chen, S-H. Evidence of the existence of the low-density liquid phase in supercooled, confined water. Proc. Natl. Acad. Sci. USA 2007, 104, 18387–18391. [Google Scholar]
- Liu, DZ; Zhang, Y; Chen, CC; Mou, CY; Poole, PH; Chen, S-H. Observation of the density minimum in deeply supercooled confined water. Proc. Natl. Acad. Sci. USA 2007, 104, 9570–9574. [Google Scholar]
- Mallamace, F; Corsaro, C; Broccio, M; Branca, C; Gonzalez-Segredo, N; Spooren, J; Chen, S-H; Stanley, HE. NMR evidence of a sharp change in a measure of local order in deeply supercooled confined water. Proc. Natl. Acad. Sci. USA 2008, 105, 12725–12729. [Google Scholar]
- Chen, S-H; Liu, L; Chu, X; Zhang, Y; Fratini, E; Baglioni, P; Faraone, A; Mamontov, E. Experimental evidence of fragile-to-strong dynamic crossover in DNA hydration water. J. Chem. Phys 2006, 125, 171103. [Google Scholar]
- Xu, L; Kumar, P; Buldyrev, SV; Chen, S-H; Poole, PH; Sciortino, F; Stanley, HE. Relation between the Widom line and the dynamic crossover in systems with a liquid-liquid phase transition. Proc. Natl. Acad. Sci. USA 2006, 102, 16558–16562. [Google Scholar]
- Sastry, S; Angell, CA. Liquid-liquid phase transition in supercooled silicon. Nat. Mater 2003, 2, 739–743. [Google Scholar]
- Morishita, T. Liquid-liquid phase transitions of phosphorus via constant-pressure first-principles molecular dynamics simulations. Phys. Rev. Lett 2001, 87, 105701. [Google Scholar]
- Saika-Voivod, I; Poole, PH; Sciortino, F. Fragile-to-strong transition and polyamorphism in the energy landscape of liquid silica. Nature 2001, 412, 514–517. [Google Scholar]
- Xu, L; Buldyrev, SV; Angell, CA; Stanley, HE. Thermodynamics and dynamics of the two-scale spherically symmetric Jagla ramp model of anomalous liquids. Phys. Rev. E 2006, 74, 031108. [Google Scholar]
- Xu, L; Buldyrev, SV; Stanley, HE. Relationship between the liquid-liquid phase transition and dynamic behaviour in the Jagla model. J. Phys. Condens. Mat 2006, 18, S2239–S2246. [Google Scholar]
- Xu, L; Mallamace, F; Yan, ZY; Starr, FW; Buldyrev, SV; Stanley, HE. Appearance of a fractional Stokes-Einstein relation in water and a structural interpretation of its onset. Nat. Phys 2009, 5, 565–569. [Google Scholar]
- Jagla, EA. Core-softened potentials and the anomalous properties of water. J. Chem. Phys 1999, 111, 8980–8986. [Google Scholar]
- Jagla, EA. A model for the fragile-to-strong transition in water. J. Phys. Cond. Matt 1999, 11, 10251–10258. [Google Scholar]
- Jagla, EA. Liquid-liquid equilibrium for monodisperse spherical particles. Phys. Rev. E 2001, 63, 061509. [Google Scholar]
- Gibson, HM; Wilding, NB. Metastable liquid-liquid coexistence and density anomalies in a core-softened fluid. Phys. Rev. E 2006, 73, 061507. [Google Scholar]
- Buldyrev, SV; Kumar, P; Debenedetti, PG; Rossky, PJ; Stanley, HE. Water-like solvation thermodynamics in a spherically symmetric solvent model with two characteristic lengths. Proc. Natl. Acad. Sci. USA 2007, 104, 20177–20182. [Google Scholar]
- Lomba, E; Almarza, NG; Martin, C; McBride, C. Phase behavior of attractive and repulsive ramp fluids: Integral equation and computer simulation studies. J. Chem. Phys 2007, 126, 244510. [Google Scholar]
- Xu, L; Buldyrev, SV; Giovambattista, N; Angell, CA; Stanley, HE. A monatomic system with a liquid-liquid critical point and two distinct glassy states. J. Chem. Phys 2009, 130, 054505. [Google Scholar]
- Franzese, G; Malescio, G; Skibinsky, A; Buldyrev, SV; Stanley, HE. Generic mechanism for generating a liquid-liquid phase transition. Nature 2001, 409, 692–695. [Google Scholar]
- Malescio, G; Franzese, G; Pellicane, G; Skibinsky, A; Buldyrev, SV; Stanley, HE. Liquid-liquid phase transition in one-component fluids. J. Phys. Condens. Mat 2002, 14, 2193–2200. [Google Scholar]
- Franzese, G; Malescio, G; Skibinsky, A; Buldyrev, SV; Stanley, HE. Metastable liquid-liquid phase transition in a single-component system with only one crystal phase and no density anomaly. Phys. Rev. E 2002, 66, 051206. [Google Scholar]
- Skibinsky, A; Buldyrev, SV; Franzese, G; Malescio, G; Stanley, HE. Liquid-liquid phase transitions for soft-core attractive potentials. Phys. Rev. E 2004, 69, 061206. [Google Scholar]
- Malescio, G; Franzese, G; Skibinsky, A; Buldyrev, SV; Stanley, HE. Liquid-liquid phase transition for an attractive isotropic potential with wide repulsive range. Phys. Rev. E 2005, 71, 061504. [Google Scholar]
- Stell, G; Hemmer, PC. Phase-transitions due to softness of potential core. J. Chem. Phys 1972, 56, 4274–4286. [Google Scholar]
- Sadr-Lahijany, MR; Scala, A; Buldyrev, SV; Stanley, HE. Liquid-state anomalies and the stell-hemmer core-softened potential. Phys. Rev. Lett 1998, 81, 4895–4898. [Google Scholar]
- Scala, A; Sadr-Lahijany, MR; Giovambattista, N; Buldyrev, SV; Stanley, HE. Waterlike anomalies for core-softened models of fluids: Two-dimensional systems. Phys. Rev. E 2001, 63, 041202. [Google Scholar]
- Scala, A; Sadr-Lahijany, MR; Giovambattista, N; Buldyrev, SV; Stanley, HE. Applications of the Stell-Hemmer potential to understanding second critical points in real systems. J. Stat. Phys 2000, 100, 97–106. [Google Scholar]
- Greaves, GN; Wilding, MC; Fearn, S; Langstaff, D; Kargl, F; Cox, S; Vu Van, Q; Majrus, O; Benmore, CJ; Weber, R; Martin, CM; Hennet, L. Detection of First-Order Liquid/Liquid Phase Transitions in Yttrium Oxide-Aluminum Oxide Melts. Science 2008, 322, 566–570. [Google Scholar]
- Bellissent-Funel, M-C. Hydration Processes in Biology: Theoretical and Experimental Approaches; ISO Press: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Robinson, GW; Zhu, S-B; Singh, S; Evans, MW. Water in Biology, Chemistry, and Physics: Experimental Overviews and Computational Methodologies; World Scientific: Singerpore, 1996. [Google Scholar]
- Workshop on “Water”: Structure and Dynamics of Water and Aqueous Solutions—Anomalies and their Possible Implications in Biology; Institute Laue Langevin: Grenoble, France, 1984.
- Debenedetti, PG. Metastable Liquids: Concepts and Principles; Princeton University Press: Princeton, NJ, USA, 1996. [Google Scholar]
- Angell, CA; Shuppert, J; Tucker, JC. Anomalous properties of supercooled water-heat-capacity, expansivity, and proton magnetic-response chemical-shit from 0 to −38 degrees. J. Phys. Chem 1973, 77, 3092–3099. [Google Scholar]
- Speedy, RJ; Angell, CA. Isothermal compressibility of supercooled water and evidence for a thermodynamic singularity at −45 degrees. J. Chem. Phys 1976, 65, 851–858. [Google Scholar]
- Maruyama, S; Wakabayashi, K; Oguni, M. Cp Maximum at 233 K for the Water within Silica Nanopores. AIP Conf. Proc 2004, 708, 675–676. [Google Scholar]
- Bergman, R; Swenson, J. Dynamics of supercooled water in confined geometry. Nature 2000, 403, 283–286. [Google Scholar]
- Faraone, A; Liu, L; Mou, C-Y; Yen, C-W; Chen, S-H. Fragile-to-strong liquid transition in deeply supercooled confined water. J. Chem. Phys 2004, 121, 10843–10846. [Google Scholar]
- Ito, K; Moynihan, CT; Angell, CA. Thermodynamic determination of fragility in liquids and a fragile-to-strong liquid transition in water. Nature 1999, 398, 492–495. [Google Scholar]
- Starr, FW; Angell, CA; Stanley, HE. Prediction of entropy and dynamic properties of water below the homogeneous nucleation temperature. Physica A 2003, 323, 51–66. [Google Scholar]
- Poole, PH; Sciortino, F; Grande, T; Stanley, HE; Angell, CA. Effect of hydrogen-bonds on the thermodynamic behavior of liquid water. Phys. Rev. Lett 1994, 73, 1632. [Google Scholar]
- Angell, CA. Water- It is a strong liquid. J. Phys. Chem 1993, 97, 6339–6341. [Google Scholar]
- Smith, RS; Kay, BD. The Existence of Supercooled Liquid Water at 150 K. Nature 1999, 398, 788–791. [Google Scholar]
- Smith, RS; Dohnalek, Z; Kimmel, GA; Stevenson, KP; Kay, BD. The Self-diffusivity of Amorphous Solid Water Near 150 K. Chem. Phys 2000, 258, 291–305. [Google Scholar]
- Velikov, V; Borick, S; Angell, CA. The glass transition of water, based on hyperquenching experiments. Science 2001, 294, 2335–2338. [Google Scholar]
- Johari, GP. Calorimetric features of high-enthalpy amorphous solids and glass-softening temperature of water. J. Chem. Phys. B 2003, 107, 9063–9070. [Google Scholar]
- Mayer, E. Water behaviour—Glass transition in hyperquenched water. Nature 2005, 435, E1. [Google Scholar]
- Stokely, K; Mazza, MG; Stanley, HE; Franzese, G. Effect of Hydrogen Bond Cooperativity on the Behavior of Water. Proc. Natl. Acad. Sci. USA 2010, 107, 1301–1306. [Google Scholar]
- Sastry, S; Debenedetti, PG; Sciortino, F; Stanley, HE. Singularity-Free Interpretation of the Thermodynamics of Supercooled Water. Phys. Rev. E 1996, 53, 6144–6154. [Google Scholar]
- Stanley, HE. A Polychromatic Correlated-Site Percolation Problem with Possible Relevance to the Unusual behavior of Supercooled H2O and D2O. J. Phys. A 1979, 12, L329–L337. [Google Scholar]
- Stanley, HE; Teixeira, J. Interpretation of The Unusual Behavior of H2O and D2O at Low Temperatures: Tests of a Percolation Model. J. Chem. Phys 1980, 73, 3404–3422. [Google Scholar]
- Poole, PH; Sciortino, F; Essmann, U; Stanley, HE. Spinodal of liquid water. Phys. Rev. E 1993, 48, 3799–3817. [Google Scholar]
- Poole, PH; Essmann, U; Sciortino, F; Stanley, HE. Phase-diagram for amorphous solid water. Phys. Rev. E 1993, 48, 4605–4610. [Google Scholar]
- Sciortino, F; Poole, PH; Essmann, U; Stanley, HE. Line of compressibility maxima in the phase diagram of supercooled water. Phys. Rev. E 1997, 55, 727–737. [Google Scholar]
- Anisimov, MA; Sengers, JV; Levelt Sengers, JMH. Aqueous System at Elevated Temperatures and Pressures: Physical Chemistry in Water, Stream and Hydrothermal Solutions; Palmer, DA, Fernandez-Prini, R, Harvey, AH, Eds.; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Levelt, JMH. Measurements of the Compressibility of Argon in the Gaseous and Liquid Phase, PhD Thesis, University of Amsterdam: Amsterdam, The Netherlands,. 1958.
- Michels, A; Levelt, JMH; Wolkers, G. Thermodynamic properties of argon at temperatures between 0 °C and −140 °C and at densities up to 640 amagat (pressures up to 1050 atmospheres). Physica 1958, 24, 769–794. [Google Scholar]
- Michels, A; Levelt, JMH; de Graaff, W. Compressibility isotherms of argon at temperatures between −25 °C and −155 °C, and at densities up to 640 amagat (pressures up to 1050 atmospheres). Physica 1958, 24, 659–671. [Google Scholar]
- Yan, ZY; Buldyrev, SV; Stanley, HE. Structural order for one-scale and two-scale potentials. Phys. Rev. Lett 2005, 95, 130604. [Google Scholar]
- Yan, ZY; Buldyrev, SV; Giovambattista, N; Debenedetti, PG; Stanley, HE. A family of tunable spherically-symmetric potentials that span the range from hard spheres to waterlike behavior. Phys. Rev. E 2006, 73, 051204. [Google Scholar]
- Yan, ZY; Buldyrev, SV; Kumar, P; Giovambattista, N; Debenedetti, PG; Stanley, HE. Structure of the first- and second-neighbor shells of simulated water: Quantitative relation to translational and orientational order. Phys. Rev. E 2007, 76, 051201. [Google Scholar]
- Yan, ZY; Buldyrev, SV; Kumar, P; Giovambattista, N; Stanley, HE. Correspondence between phase diagrams of the TIP5P water model and a spherically symmetric repulsive ramp potential with two characteristic length scales. Phys. Rev. E 2008, 77, 042201. [Google Scholar]
- Yan, ZY; Buldyrev, SV; Stanley, HE. Relation of water anomalies to the excess entropy. Phys Rev E 2008, 78, 051201. [Google Scholar]
- Errington, JR; Debenedetti, PG. Relationship between structural order and the anomalies of liquid water. Nature 2001, 409, 318–321. [Google Scholar]
- Hemmati, M; Moynihan, CT; Angell, CA. Interpretation of the molten BeF2 viscosity anomaly in terms of a high temperature density maximum, and other waterlike features. J. Chem. Phys 2001, 115, 6663–6671. [Google Scholar]
- Corradini, D; Buldyrev, SV; Gallo, P; Stanley, HE. Effect of hydrophobic solutes on the liquid-liquid critical point. Phys. Rev. E 2010, 81, 061504. [Google Scholar]
- Molinero, V; Sastry, S; Angell, CA. Tuning of tetrahedrality in a silicon potential yields a series of monatomic (metal-like) glass formers of very high fragility. Phys. Rev. Lett 2006, 97, 075701. [Google Scholar]
- Loerting, T; Giovambattista, N. Amorphous ices: Experiments and numerical simulations. J. Phys. Condens. Matter 2006, 18, R919–R977. [Google Scholar]
- Poole, PH; Saika-Voivod, I; Sciortino, F. Density minimum and liquid-liquid phase transition. J. Phys. Condens. Matter 2005, 17, L431–L437. [Google Scholar]
- Paschek, D. How the Liquid-Liquid Transition Affects Hydrophobic Hydration in Deeply Supercooled Water. Phys. Rev. Lett 2005, 94, 217802. [Google Scholar]
- Tver’yanovich, LS; Ushakov, VM; Tverjanovich, A. Heat of structural transformation at the semiconductor-metal transition in As2Te3 liquid. J. Non-Cryst. Solids 1996, 197, 235–237. [Google Scholar]
- Tsuchiya, Y. The molar volume of molten As-Sb, As-Bi and As-Te systems: further evidence for rapid structural changes in liquid As in the supercooled state. J. Non-Cryst. Solids 1999, 250, 473–477. [Google Scholar]
- Sen, S; Andrus, RL; Baker, DE; Murtagh, MT. Observation of an Anomalous Density Minimum in Vitreous Silica. Phys. Rev. Lett 2004, 93, 125902. [Google Scholar]
- Oguni, M; Angell, CA. Anomalous components of supercooled water expansivity, compressibility, and heat-capacity (CP and Cv) from binary formamide+water solution studies. J. Chem. Phys 1983, 78, 7334–7342. [Google Scholar]
- Aptekar, IL; Ponyatovskii, YG. Theory of cerium isomorphism .I. Equilibrium P – T phase diagram. Phys. Met. Metall 1968, 25, 10. [Google Scholar]
- Zhang, B; Wang, RJ; Wang, WH. Response of acoustic and elastic properties to pressure and crystallization of Ce-based bulk metallic glass. Phys. Rev. B 2005, 72, 104205. [Google Scholar]

© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, L.; Buldyrev, S.V.; Giovambattista, N.; Stanley, H.E. Liquid-Liquid Phase Transition and Glass Transition in a Monoatomic Model System. Int. J. Mol. Sci. 2010, 11, 5184-5200. https://doi.org/10.3390/ijms11125184
Xu L, Buldyrev SV, Giovambattista N, Stanley HE. Liquid-Liquid Phase Transition and Glass Transition in a Monoatomic Model System. International Journal of Molecular Sciences. 2010; 11(12):5184-5200. https://doi.org/10.3390/ijms11125184
Chicago/Turabian StyleXu, Limei, Sergey V. Buldyrev, Nicolas Giovambattista, and H. Eugene Stanley. 2010. "Liquid-Liquid Phase Transition and Glass Transition in a Monoatomic Model System" International Journal of Molecular Sciences 11, no. 12: 5184-5200. https://doi.org/10.3390/ijms11125184



