1. Introduction
We previously showed that oxidized phosphatidylcholine (oxPtdPC) is chemically linked to naturally occurring human apolipoprotein (a) [apo(a)] and that this linkage involves 1 to 2 lysines in kringle V located in the
C-terminal domain of this apolipoprotein [
1]. We subsequently showed that oxPtdPCs are linked to apo(a) in a 2:1 ratio that is not affected by plasma Lp(a) levels or apo(a) size polymorphisms [
2]. We also reported that in healthy subjects, linked oxPtdPCs do not originate from plasma LDL or the LDL component of Lp(a) [
2]. Recently, we reported that naturally occurring human Plg, a multi-kringle structure that shares structural and genetic features with apo(a), contains about 2 mol of oxPtdPCs/mol [
3]. We also showed that human HepG2 cells secrete Plg containing linked oxPtdPCs, [
3] suggesting that these modified lipids are covalently linked to Plg in the liver [
2].
The purpose of the present study was to determine whether mouse Plg, which is 79% homologous to human Plg, contains linked oxPtdPCs and whether these oxidized lipid species are subject to metabolism by lipoprotein-associated phospholipase A
2 (Lp-PLA
2) [
4]. This enzyme, also known as platelet-activating factor (PAF) acetylhydrolase (PAF-AH) has specificity for hydrolysis of
sn-2 acyl groups present in biologically active phospholipids such as PAF and oxPtdPCs, and has been proposed to have both pro- and anti-inflammatory functions [
5]. Previous studies were focused on the ability of Lp-PLA
2 to hydrolyze free and non-covalently-bound, lipoprotein-associated oxPtdPCs in human [
6] and mouse [
7,
8] plasma. Thus, the issue of whether Lp-PLA
2 metabolizes oxPtdPC-protein adducts, particularly in
in vivo settings, is a novel area of investigation.
3. Experimental Section
3.1. Materials and Methods
The studies in mice were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the committee on the Ethics of Animal Experiments of the University of UTAH (animal welfare assurance number A3031-01).
3.2. Materials
BSA, Tween-20, SDS, ε-amino caproic acid, 4-(2-Aminoethyl)-benzene sulfonylfluoride, and N-α-tosyl-L-lysine chloromethylketone hydrochloride were from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Immobilon-P membranes were from Millipore Corp. (Billerica, MA, USA) and Superblock blocking buffer and the Supersignal® West Dura Extended Duration Substrate were purchased from Thermo Scientific (Rockford, IL). Coomassie staining solution (Page Blue) was from Fermentas Inc. (Glen Burnie, MD, USA). Precast acrylamide gels were from Invitrogen Corp. (Carlsbad, CA, USA). All chemicals were of reagent grade.
Human recombinant Lp-PLA2 (Pafase®) was a generous gift from ICOS Corporation (Bothell, WA, USA).
3.3. Antibodies
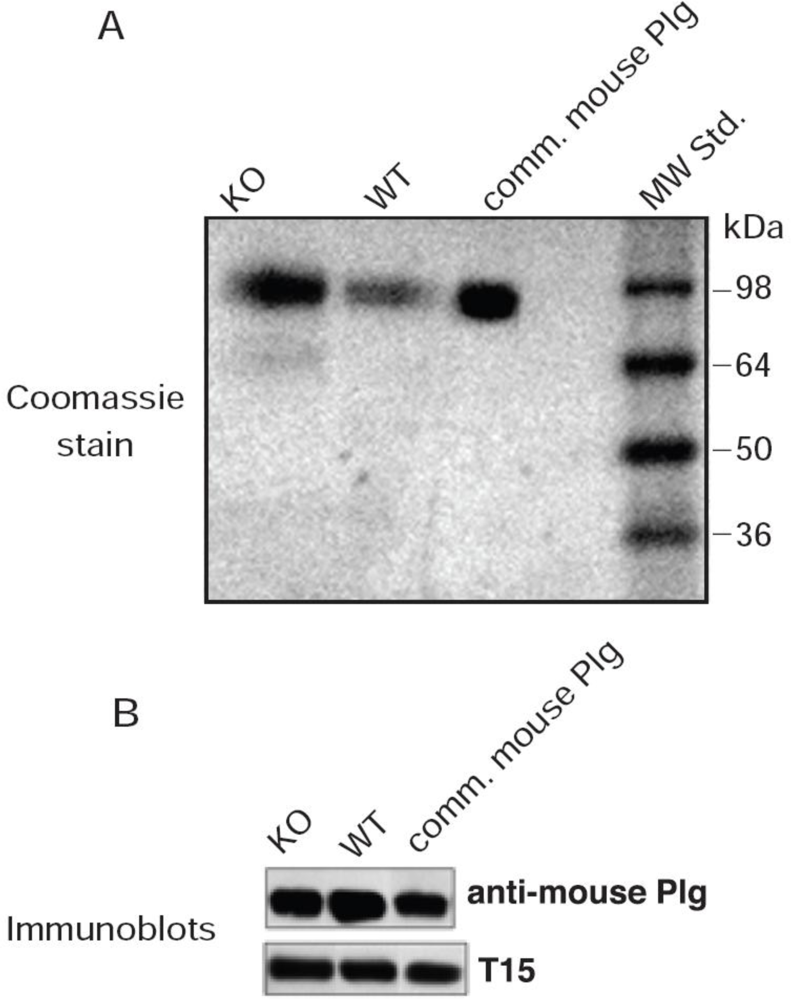
The murine T15 antibody-secreting cell line, BH8 (IgM) that reacts with oxPtdPC antigens was a gift from Dr. John F. Kearney (University of Alabama) and is heretofore referred to as T15. This cell line was maintained in the Frank W. Fitch Antibody Facility of the University of Chicago. The antibodies in the culture medium were isotyped and contained a high titer of IgM antibodies (greater than 90%) as verified by the major Coomassie stained band on SDS-PAGE.
Affinity purified rabbit anti-mouse Plg polyclonal antibodies were obtained from Cell Sciences (Canton, MA, USA). Horseradish peroxidase (HRP)-labeled secondary antibodies [goat anti-mouse IgM (μ chain specific) and donkey anti-rabbit IgG] were from Sigma-Aldrich.
3.4. Plasminogen Isolation and Quantitation
Plasma from healthy mice was utilized to purify Plg using the procedure of Deutsch and Mertz [
9], followed by purification on G25 Sepharose columns. We also employed commercial preparations of mouse Plg purchased from Enzyme Research Laboratories (South Bend, IN, USA). Quantitative determination of Plg in mouse plasma and in purified samples was conducted using an ELISA kit (Kamiya Biomedical Co., Seattle, WA, USA). The Plg standard concentrations ranged from 6.25 to 200 ng/mL.
3.5. Delipidation of Plasminogen
Lipids were extracted from purified Plg by a modified Bligh and Dyer method [
10] in that chloroform was replaced by anhydrous diethyl ether in a ratio of 1:2 v/v (ether:methanol). Plg in 0.8 mL 50 mM Tris-HCl containing 0.1 M NaCl, pH 7.4 was added to the ethyl ether-methanol mixturedropwise and rotated at 4 °C for 6 h. The mixture was then centrifuged and the precipitated protein washed three times with diethyl ether. The washed precipitate was dried with argon gas and dissolved in the Tris buffer. The protein recovery was close to 85% of the original starting material. An alternative delipidation technique was also carried out using a 3:2 v/v mixture of ethanol:diethyl ether as already published for apo(a) [
1]. In both delipidation techniques the Plg was soluble in aqueous buffers.
3.6. Electrophoretic Methods
SDS-PAGE (gradients of 4–12% polyacrylamide) was performed on a Novex system (Novex, San Diego, CA) for 1.5 h at constant voltage (120 V) at 22 °C, as described [
1,
2]. The samples were prepared by heating at 95 °C for 5 min in sample buffer [94 mM phosphate buffer (pH 7.0), 1% SDS and 2 M urea, with or without 3% mercaptoethanol]. Following electrophoresis, the gels were placed on Immobilon-P sheets (Millipore Corp., Bedford, MA) previously wetted in 48 mM Tris, 39 mM glycine (pH 8.9). Blotting was performed on a horizontal semi-dry electroblot apparatus (Amersham Biosciences) at 0.8–1 mA/cm
2 for 45 min at 23 °C.
3.7. Immunoblot Analyses
After electroblotting, the Immobilon-P sheets were blocked in Superblock (Thermo Scientific, Inc. Rockford, IL. USA) for 1 h at 23 °C followed by incubation with rabbit anti-mouse Plg IgG or, in the case of T15, by incubation in 10% Superblock for 18 h at 4 °C. Bound antibodies were visualized using secondary antibodies conjugated to HRP; donkey anti-rabbit IgG for Plg, and goat anti-mouse IgM (μ-chain specific) for oxPtdPC. The blots were developed with Supersignal® West Dura Extended Duration Substrate from Thermo Scientific, Inc. (Rockford, IL).
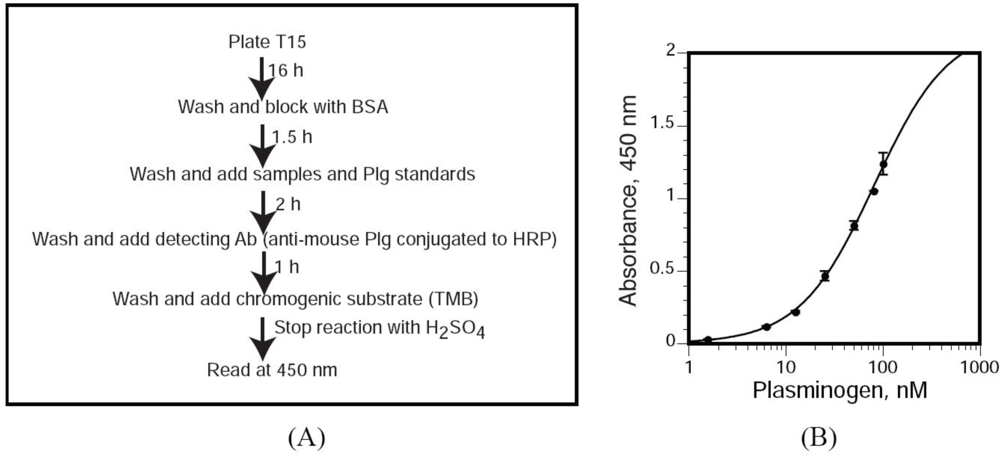
3.8. T15 ELISA for Quantitation of OxPtdPC
We utilized a novel sandwich ELISA by which we measured T15 immunoreactivity in Plg isolated from mouse plasma. Polystyrene microtiter plates (flat-bottom 96-well EIA plates) were coated with 100 μL of T15 [400 ng/well, dissolved in TBS buffer [50 mmol/L Tris-HCl, 0.15 mol/L NaCl (pH 7.5)] and incubated overnight at room temperature, in 10 mmol/L Tris, 0.15 mol/L NaCl (pH 7.6). Unbound antibodies were removed by washing with TBS supplemented with 0.1% BSA and 0.02% Tween-20. Non-specific binding sites were blocked with 1% BSA in TBS for 1.5 h. After three washes with TBS supplemented with 0.02% Tween-20 (TBST), we added 100 μL of each dilution of the mouse Plg standard and samples (diluted in TBST) and incubated the plates for 2 h at room temperature. After three washes with TBST, bound Plg was detected by incubation with a specific, HRP-conjugated anti-mouse Plg polyclonal antibody for 1 h in TBST. The wells were washed three times with TBST, the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine was added and, after an appropriate incubation period at room temperature, the reactions were stopped with 3 mol/L H2SO4. T15 immunoreactivity was quantitated by assessing the absorbance at 450 nm using a Versamax microplate reader (Molecular Devices, Sunnyvale, CA). The concentrations of the Plg standard ranged from 1.56 to 100 nmol/L.
3.9. Defining T15 Equivalents
Based on the standard curve, the absorbance of each sample reacting with T15 was converted to nmol/L of Plg. This number was divided by the concentration (nmol/L) of Plg in each mouse sample. This ratio was defined as the “T15 equivalent”.
3.10. Targeted Disruption of the Mouse Lp-PLA2 (Pla2g7) Gene
The generation of
Pla2g7−/− mice took place at the University of Utah and was recently described in detail [
11] Briefly, a targeting vector lacking exons 3 and 4 of the mouse
Pla2g7 gene was introduced into embryonic stem cells and a resulting chimeric male was bred to C57BL/6J females. The progeny was genotyped as previously described [
11].
3.11. Incubation of Mouse Plg with Lp-PLA2
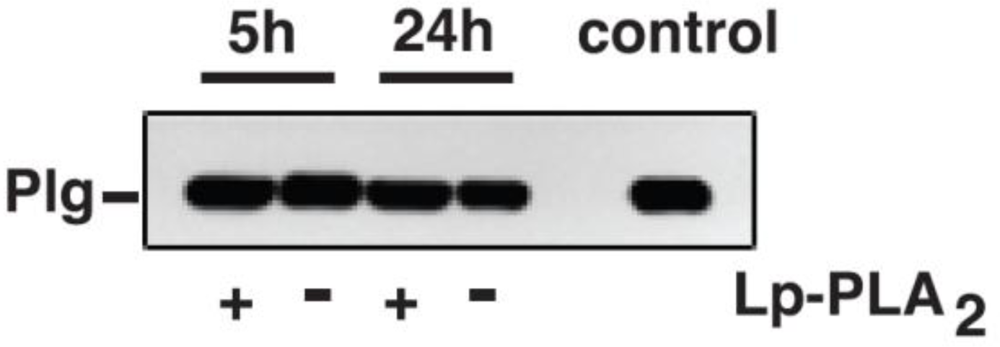
Mouse Plg (1 to 3 μg) was incubated with recombinant Lp-PLA2 (4 μg) in PBS for up to 24 h at 37 °C. The reactions were stopped with Pefabloc and the samples were stored frozen at −20 °C until subsequent analyses.
4. Discussion
A novel finding of our current studies was that freshly isolated mouse Plg and commercial sources contain chemically linked oxPtdPCs. The chemical linkage, likely of the Schiff base nature, was suggested by the observations that T15 immunoreactivity was present in fresh and frozen samples, was not altered by extensive delipidation with organic solvents or by boiling in the presence of SDS. Of note, our observations were made under basal, unstimulated conditions, and paralleled our previous results in both apo(a) and Plg isolated from normal human plasma [
2,
3]. Together, our findings challenge the belief that oxPtdPC generation only occurs in response to pro-oxidant/pro-inflammatory events which would presumably be quite limited in healthy subjects and in unstimulated mice [
4]. A plausible explanation is that adduct formation occurs during early, pre-secretory stages of Plg generation. In previous studies we proposed a model whereby the syntheses of human apo(a) [
1] and human Plg [
3] may be accompanied, or shortly followed, by oxPtdPC linkage to newly synthesized proteins. We now speculate that the liver provides a minimally pro-oxidant/pro-inflammatory microenvironment exquisitely sensed by a lysine residue(s) in the Plg molecule, leading to the formation of oxPtdPC-Plg adducts.
A second novel observation from quantitative and metabolic standpoints was that the extent of Plg conjugation with oxPtdPC was not affected by Lp-PLA
2. This suggests that under basal conditions, Lp-PLA
2 does not play a critical role in the metabolism of Plg-bound oxPtdPCs during transport in the circulation. Lack of activity may be due to peculiar Plg conformations that prevent the candidate linkage sites from interacting with Lp-PLA
2, an interpretation consonant with
in vitro studies in which oxPtdPC was not cleaved when mouse Plg was incubated with Lp-PLA
2. We recognize that in our
in vitro studies we utilized a recombinant rather than a naturally occurring Lp-PLA
2 that, in the mouse plasma, under normal conditions is expected to be essentially all bound to HDL [
4]. We believe, however, that the validity of our
in vitro experiments is supported by the results of our
in vivo studies in KO mice. In our previous study, cleavage of oxPtdPCs linked to human Plg occurred but only under conditions of a high enzyme to substrate ratio and prolonged times of incubation [
3].
Further studies are needed to determine whether the number of oxPtdPCs linked to Plg increases in settings of elevated oxidant and/or inflammatory stress and to assess whether Lp-PLA
2 modulates this process. These analyses may provide important clues to explain why Plg has pathological cardiovascular properties, an issue emerging from recent studies in Plg-deficient mice [
12]. In this regard, plasma from transgenic mice expressing human apo(a) contain Lp(a)/apo(a) with bound oxPtdPCs proposed to contribute to uremia-induced atherosclerosis [
13,
14]. However, the potential contribution by the oxPtdPC-Plg adducts cannot be ruled out. Considering our observation that Plg is the major oxPtdPC carrier in the circulation, contrary to an early belief [
15], we may speculate that Plg modulates OxPtdPC metabolism in the circulation, in physiological and possibly pathological settings.
In the current and previous studies, the number of linked oxPtdPCs was limited to 1 or 2. In the case of apo(a), the modification involved specific microdomains within kringle V [
1,
3], suggesting that a kringle repeat(s) may also participate in linkage of OxPtdPCs to Plg. Under physiological conditions it is possible that the formation of Plg adducts reflects a mechanism to shield potential pro-inflammatory OxPtdPCs from metabolism during transport in the circulation. To rigorously test this, it would be ideal to compare the properties of Plg adducts harboring various amounts of oxPtdPCs. Unfortunately, given the chemical nature of the oxPtdPC-Plg linkage, the lipid component is not amenable to extraction by organic solvents; drastic procedures would likely result in denatured, unsuitable bio-products. Also unfortunate is the fact that Lp-PLA
2 cannot be utilized to generate OxPtdPC-free Plg. Mutagenic approaches should prove useful, but it will be necessary to first identify the oxPtdPC linkage site on Plg.
5. Conclusions
In conclusion, under physiological conditions, mouse Plg, like its human counterpart, carries chemically linked oxPtdPCs that are not cleaved by Lp-PLA2, an enzyme known to be active on oxidized phospholipids associated with plasma lipoproteins. Future studies will elucidate the molecular basis for lack of recognition and/or metabolism of Plg-bound oxPtdPCs by Lp-PLA2. These studies should provide a platform to assess possible relationships between oxPtdPCs, Plg, and Lp-PLA2 under stressed and pathological conditions. Work along these lines may have broad physio-pathological implications, including issues related to the function of the pro-atherogenic lipoprotein Lp(a).