Colon-Specific Drug Delivery Behavior of pH-Responsive PMAA/Perlite Composite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analyses of Extent Interactions between Organic and the Inorganic Moieties
2.2. Thermal Behavior
2.3. Swelling of Composite
2.4. Release Studies
3. Experimental
3.1. Materials
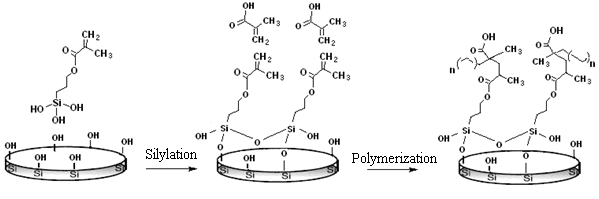
3.2. Silylation
3.3. Graft Polymerization
3.4. The Swelling Ratio of Matrices
3.5. Drug Loading in Composites
3.6. Determination of the Amount of Drug Entrapped
3.7. Characterization of Hydrolysis Product
3.8. In Vitro Drug Release Study
4. Conclusions
Acknowledgments
References
- Celis, R; Hermosín, MC; Cornejo, J. Heavy metal adsorption by functionalized clays. Environ. Sci. Technol 2000, 34, 4593–4599. [Google Scholar]
- Guimarãesa, A; Ciminellib, V; Vasconcelosb, W. Surface modification of synthetic clay aimed at biomolecule adsorption: Synthesis and characterization. Mater. Res 2007, 10, 37–41. [Google Scholar]
- He, H; Duch, J; Galy, J; Gerard, JF. Grafting of swelling clay materials with 3- aminopropyltriethoxysilane. J. Colloid Interface Sci 2005, 288, 171–176. [Google Scholar]
- Patil, AJ; Muthusamy, E; Mann, S. Fabrication of functional protein- or-ganoclay lamellar nanocomposites by biomolecule-induced assembly of exfoliated aminopropyl-functionalized magnesium phyllosilicates. J. Mater. Chem 2005, 15, 3838–3843. [Google Scholar]
- Kuz′niarska-Biernacka, I; Silva, AR; Carvalho, AP; Pires, J; Freire, C. Organo-laponites as novel mesoporous supports for manganese (iii) salen catalysts. Langmuir 2005, 21, 10825–10834. [Google Scholar]
- Walcarius, A; Etienne, M; Delacote, C. Uptake of inorganic Hg (II) by organically modified silicates: Influence of pH and choride concentration on the binding pathways and electrochemical monitoring of the processes. Anal. Chim. Acta 2004, 508, 87–98. [Google Scholar]
- Bois, L; Bonhommé, A; Ribes, A; Pais, B; Raffin, G; Tessier, F. Functional-ized silica for heavy metal ions adsorption. Colloid Surface Physicochem. Eng. Aspects 2003, 221, 221–230. [Google Scholar]
- Mansur, HS; Vasconcelos, WL; Lenza, RFS; Oréfice, RL; Reis, EF; Lobato, ZP. Sol-Gel silica based networks with controlled chemical properties. J. Non-Cryst. Solids 2000, 273, 109–115. [Google Scholar]
- Wang, B; Smith, T. Performance of a diatomite-based sorbent in removing mercury from aqueous and oil matrices. J. Environ. Eng. Sci 2007, 6, 469–476. [Google Scholar]
- Mercier, L; Detellier, C. Preparation, Characterization and Applications as Heavy metals sorbents of covalently grafted thiol functionalities on the interlamelar surface of montmorillonite. Environ. Sci. Technol 1995, 29, 1318–1323. [Google Scholar]
- Sayilkan, H; Erdemoglu, S; Sener, S; Sayilkan, F; Akarsu, M; Erdemo-glu, M. Surface modification of pyrophyllite with amino silane coupling agent for the removal of 4-nitrophenol from aqueous solutions. J. Colloid Interface Sci 2004, 275, 530–538. [Google Scholar]
- Park, KW; Kwon, OY. Inter lamellar silylation of montmorillonite with 3-aminopropyl-triethoxysilane. Bull. Korean Chem. Soc 2004, 25, 965–968. [Google Scholar]
- Alkan, M; Do, M. Surface titrations of perlite suspentions. J. Colloid Interface Sci 1998, 207, 90–96. [Google Scholar]
- Bai, YX; Li, YF; Yang, Y; Yi, LX. Covalent immobilization of triacylglycerol lipase onto functionalized novel mesoporous silica supports. J. Biotechnol 2006, 125, 574–582. [Google Scholar]
- Zhoa, D; Feng, J; Huo, Q; Melosh, N; Fredrickson, GH; Chmelka, BF; Stucky, GD. Triblock copolymer syntheses of mesoporous silica with periodic 50–300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar]
- Lin, CC; Yang, MC. Cholesterol oxidation using hollow fiber dialyzer immobilized with cholesterol oxidase: Preparation and properties. Biotechnol. Progr 2003, 19, 361–364. [Google Scholar]
- Nguyen, V; Yoshida, W; Jou, JD; Cohen, YJ. Kinetics of free-radical graft polymerization of 1-vinyl-2-pyrrolidone onto silica. J. Polym. Sci. Part A: Polym. Chem 2002, 40, 26–42. [Google Scholar]
- Tozaki, H; Odoriba, T; Okada, N; Fujita, T; Terabe, A; Suzuki, T; Okabe, S; Muranishi, S; Yamamoto, A. Chitosan capsules for colon-specific drug delivery: Enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J. Control. Rel 2002, 82, 51–61. [Google Scholar]
- Mahkam, M; Doostie, L. The relation between swelling properties and cross-linking of hydrogels designed for colon-specific drug delivery. Drug Delivery 2005, 12, 343–347. [Google Scholar]
- Gulsen, D; Chauhan, A. Effect of water content on transparency, swelling, lidocaine diffusion in p-HEMA gels. J. Membr. Sci 2006, 269, 35–48. [Google Scholar]
- Fanovich, MA; Toledano, M. Dental composites reinforced with hydroxyapatite: Mechanical behavior and absorption/elution characteristics. J. Biomed. Mater. Res 2001, 56, 297–305. [Google Scholar]
- Thwe, MM; Liao, K. Durability of bambooglass fiber reinforced polymer matrix composite composites. Compos. Sci. Technol 2003, 63, 375–387. [Google Scholar]
- Arbelaiz, A; Fernández, B; Ramos, JA; Retegi, A; Llano-Ponte, R; Mondragon, I. Mechanical properties of short flax fibre bundle/polypropylene composites: Influence of matrix/fibre modification, fibre content, water uptake and recycling. Compos. Sci. Technol 2005, 65, 1582–1592. [Google Scholar]
- Lara, MG; Vitória, M; Bentley, LB; Collett, JH. In vitro drug release mechanism and drug loading studies of cubic phase gels. Int. J. Pharm 2005, 293, 241–250. [Google Scholar]
- Baldwin, SP; Saltzman, WM. Materials for protein delivery in tissue engineering. Adv. Drug Delivery Rev 1998, 33, 71–86. [Google Scholar]
- Rubinstein, A. Natural polysaccharides as targeting tools of drugs to the human colon. Drug Discovery Dev 2000, 50, 435–439. [Google Scholar]
- Mahkam, M. Controlled release of biomolecules from pH-sensitive hydrogels by radiation polymerization. J. Bioact. Comp. Polym 2004, 19, 209–220. [Google Scholar]
- Bajpai, SK; Saxena, SJ. Enzymatically degradable and pH-sensitive hydrogels for colon-targeted oral drug delivery. I. Synthesis and characterization. J. Appl. Polym. Sci 2004, 92, 3630–3643. [Google Scholar]
- Mahkam, M. New pH-sensitive glycopolymers for colon-specific drug delivery. Drug Delivery 2007, 14, 147–153. [Google Scholar]
- Mahkam, M. New terpolymers as hydrogels for oral protein delivery application. J. Drug Targeting 2009, 17, 29–35. [Google Scholar]
- Geresh, S; Gilboa, Y; Peisahov-Korol, J; Gdalevsky, G; Voorspoels, J; Remon, JP; Kost, JJ. Preparation and characterization of bioadhesive grafted starch copolymers as platforms for controlled drug delivery. Appl. Polym. Sci 2002, 86, 1157–1162. [Google Scholar]
- Kopeček, J; Kopečková, P; BrØndsted, H; Rathi, R; Řihoá, B; Yeh, PY; Ikesue, K. Polymers for colon-specific drug delivery. J. Control. Release 1992, 19, 121–130. [Google Scholar]


| Organic/inorganic composite | Molar composition in the feed (%) | |
|---|---|---|
| TSPA | MAA | |
| APC-1 | 5 | 10 |
| APC-2 | 5 | 30 |
| APC-3 | 10 | 10 |
| APC-4 | 10 | 30 |
| Organic/inorganic composite | Tg (°C) | Residue (%) |
|---|---|---|
| APC-1 | 150 | 97 |
| APC-2 | 162 | 93 |
| APC-3 | 180 | 88 |
| APC-4 | 195 | 83 |
| Composites | Maximum constant swelling (%) pH 1 | Maximum constant swelling (%) pH 7.4 | Percent of 5-ASA-loading (%) |
|---|---|---|---|
| APC-1 | 8 | 26 | 87 |
| APC-2 | 5 | 35 | 96 |
| APC-3 | 4 | 22 | 78 |
| APC-4 | 2 | 30 | 90 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mahkam, M.; Vakhshouri, L. Colon-Specific Drug Delivery Behavior of pH-Responsive PMAA/Perlite Composite. Int. J. Mol. Sci. 2010, 11, 1546-1556. https://doi.org/10.3390/ijms11041546
Mahkam M, Vakhshouri L. Colon-Specific Drug Delivery Behavior of pH-Responsive PMAA/Perlite Composite. International Journal of Molecular Sciences. 2010; 11(4):1546-1556. https://doi.org/10.3390/ijms11041546
Chicago/Turabian StyleMahkam, Mehrdad, and Laleh Vakhshouri. 2010. "Colon-Specific Drug Delivery Behavior of pH-Responsive PMAA/Perlite Composite" International Journal of Molecular Sciences 11, no. 4: 1546-1556. https://doi.org/10.3390/ijms11041546