Functionality and Antioxidant Properties of Tilapia (Oreochromis niloticus) as Influenced by the Degree of Hydrolysis
Abstract
:1. Introduction
2. Results and Discussion
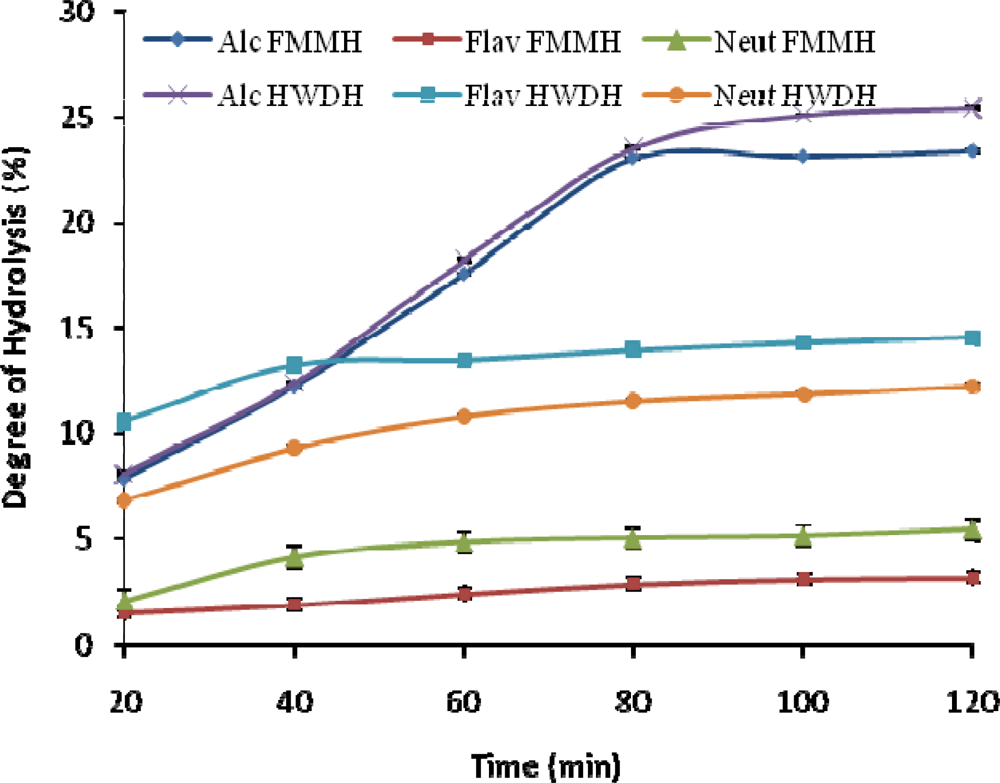
2.1. Degree of Hydrolysis (DH)
2.2. Amino Acid Analysis
2.3. Molecular Weight Distribution
2.4. DPPH Radical Scavenging Activity
2.5. ABTS Radical Scavenging Activity
2.6. Metal-Chelating Activity
2.7. Nitrogen Solubility
2.8. Oil/Water Holding Capacity
2.9. Emulsifying Capacity (EC)
2.10. Foam Capacity and Foam Stability (FC/FS)
2.11. Bulk Density
2.12. In Vitro Protein Digestibility
3. Experimental
3.1. Materials and Methods
3.2. Preparation of Fish Protein Hydrolysates
3.3. Determination of the Degree of Hydrolysis DH
3.4. Amino Acid Analysis
3.5. Determination of Molecular Weight
3.6. DPPH Radical Scavenging Activity Assay
3.7. ABTS Radical Scavenging Activity Assay
3.8. Metal-Chelating Activity
3.9. Nitrogen Solubility (NS)
3.10. Oil-Holding Capacity (OHC)
3.11. Water-Holding Capacity (WHC)
3.12. Emulsifying Capacity (EC)
3.13. Foaming Capacity (FC) and Foam Stability (FS)
3.14. Bulk Density (BD)
3.15. In Vitro Protein Digestibility (IVPD)
3.16. Statistical Analysis
4. Conclusions
Acknowledgments
References
- El-Sayed, AM. Tilapia Culture; CABI publishing: Oxford, UK, 2006; pp. 1–24. [Google Scholar]
- Hempel, E. Tilapia the new white fish. Seafood Int 2002, 10, 16–23. [Google Scholar]
- National Nutrient Database for Standard Reference, USDA: Washington, DC, USA; Release No. 18, 2005. Available at: www.nal.usda.gov/fnic/foodcomp/Data (Accessed on 12 April 2010).
- Chobert, JM; Bertrand-Harb, C; Nicolus, MG. Solubility and emulsifying properties of caseins and whey proteins modified enzymatically by trypsin. J. Agric. Food Chem 1988, 36, 883–892. [Google Scholar]
- Panyam, D; Kilara, A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci. Technol 1996, 7, 120–125. [Google Scholar]
- Shahidi, F; Han, XQ; Synowiecki, J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem 1995, 53, 285–293. [Google Scholar]
- Gbogouri, GA; Linder, M; Fanni, J; Parmentier, M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J. Food Sci 2004, 69, 615–622. [Google Scholar]
- Sathivel, S; Smiley, S; Prinyawiwatkul, W; Bechtel, PJ. Functional and nutritional properties of red salmon (Oncorhynclnis nerka) enzymatic hydrolysates. J. Food Sci 2005, 70, 401–406. [Google Scholar]
- Diniz, FM; Martin, AM. Effects of the extent of enzymatic hydrolysis on the functional properties of shark protein hydrolysate. LWT- Food Sci. Technol 1997, 30, 266–272. [Google Scholar]
- Sathivel, S; Bechtel, PJ; Babbitt, J; Smiley, S; Crapo, C; Reppond, KD. Biochemical and functional properties of herring (Clupea harengus) byproduct hydrolysates. J. Food Sci 2003, 68, 2196–2200. [Google Scholar]
- Quaglia, GB; Orban, E. Influence of enzyrnatic hydrolysis on structure and emulsifying properties of sardine (Sardina pilchardus) protein hydrolysates. J. Food Sci 1990, 55, 1571–1573. [Google Scholar]
- Quaglia, GB; Orban, E. Influence of the degree of hydrolysis on the solubility of the protein hydrolysates from sardine (Sardina pilchardus). J. Sci. Food Agric 1987, 38, 271–276. [Google Scholar]
- Amarowicz, R; Shahidi, F. Antioxidant activity of peptide fractions of capelin protein hydrolysates. Food Chem 1997, 58, 355–359. [Google Scholar]
- Wu, HC; Chen, HM; Shiau, CY. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int 2003, 36, 949–957. [Google Scholar]
- Alasalvar, C; Shahidi, F; Quantick, P. Food and health applications of marine neutraceuticals. In Seafoods–Quality Technology and Nutraceutical Applications; Alasalvar, C, Taylor, T, Eds.; Springer-Verlag: Berlin, Germany, 2002; pp. 175–204. [Google Scholar]
- Hagen, H; Sandnes, K. Process for improvement of meat quality in fish, protein hydrolysate and method of producing a protein hydrolysate. 2004. [Google Scholar]
- Guerard, F; Guimas, A; Binet, J. Production of tuna waste hydrolysates by a commercial neutral protease preparation. J. Mol. Catal. B: Enzym 2002, 19, 489–498. [Google Scholar]
- FAO Protein and Amino Acid Requirements in Human Nutrition, Report of a joint WHO/FAO/UNU expert consultation, WHO technical report series, No 935. The World Health Organization: Geneva, Switzerland, 2007.
- You, L; Zhao, M; Cui, C; Zhao, H; Yang, B. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innov. Food Sci. Emerg. Technol 2009, 2, 235–240. [Google Scholar]
- Yang, B; Zhao, MM; Shi, J; Yang, N; Jiang, YM. Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chem 2008, 106, 685–690. [Google Scholar]
- Jao, CL; Ko, WC. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging by protein hydrolyzates from tuna cooking juice. Fish Sci 2002, 68, 430–435. [Google Scholar]
- Kim, SK; Kim, YT; Byun, HG; Nam, KS; Joo, DS; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska Pollock skin. J. Agric. Food Chem 2001, 49, 1984–1989. [Google Scholar]
- Miliauskasa, G; Venskutonisa, PR; Beek, TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem 2004, 85, 231–237. [Google Scholar]
- Klompong, V; Benjakul, S; Kantachote, D; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem 2007, 102, 1317–1327. [Google Scholar]
- Saiga, A; Tanabe, S; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrilar proteins by protease treatment. J. Agric. Food Chem 2003, 51, 3661–3667. [Google Scholar]
- Sorgentini, DA; Wagner, JR. Comparative study of foaming properties of whey and isolate soy bean proteins. Food Res. Int 2002, 35, 721–729. [Google Scholar]
- Petruccelli, S; Anon, MC. Soy protein isolates components and their interactions. J. Agric. Food Chem 1994, 43, 1762–1767. [Google Scholar]
- Kuehler, CA; Stine, CM. Effect of enzymatic hydrolysis on some functional properties of whey proteins. J. Food Sci 1974, 39, 379–382. [Google Scholar]
- Wasswa, J; Tang, J; Gu, X. Functional properties of Grass carp (Ctenopharyngodon idella), Nile perch (Lates niloticus) and Nile tilapia (Oreochromis niloticus) skin hydrolysates. Int. J. Food Prop 2008, 11, 339–350. [Google Scholar]
- Kamara, MT; Zhu, K; Issoufou, A; Tarawalie, F; Zhou, H. Functionality, in vitro digestibility and physicochemical properties of two varieties of defatted foxtail millet protein concentrates. Int. J. Mol. Sci 2009, 10, 5224–5238. [Google Scholar]
- Azizah, AH; Bakar, J; Bee, GH. Nutritional quality of spray dried protein hydrolysate from Black Tilapia (Oreochromis mossambicus). Food Chem 2002, 78, 69–74. [Google Scholar]
- Van DER Plancken, I; Van Remoortere, M; Van Loey, A; Hendrickx, ME. Heat induced changes in the susceptibility of egg white proteins to enzymatic hydrolysis: A kinetic study. J Agric Food Chem 2003, 51, 3819–3823. [Google Scholar]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: New York, NY, USA, 1986. [Google Scholar]
- Moore, S. On the determination of cystine and cysteic acid. J. Biol. Chem 1963, 238, 235–237. [Google Scholar]
- Ravindran, G; Bryden, WL. Tryptophan determination in proteins and feedstuffs by ion exchange chromatography. Food Chem 2005, 89, 309–314. [Google Scholar]
- Re, R; Pellegrini, N; Proteggente, A; Pannala, A; Yang, M; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med 1999, 26, 1231–1237. [Google Scholar]
- Decker, EA; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem 1990, 38, 674–677. [Google Scholar]
- Rakesh, J; Metz, A. Acid precipitated fish protein isolate exhibits good functional properties. Food Product. Dev 1973, 7, 18–24. [Google Scholar]
- Bernardi Don, LS; Pilosof, AMR; Bartholomai, GB. Enzymatic modification of soy protein concentrates by fungal and bacterial proteases. J. Am. Oil Chem. Soc 1991, 68, 102–105. [Google Scholar]
- Elkhalil, EAJ; El Tinay, AH; Mohamed, BE; Elshseikh, EAE. Effect of malt pretreatment on phytic acid and in vitro protein digestibility of sorghum flour. Food Chem 2001, 72, 29–32. [Google Scholar]


| Amino Acids | Alcalase 2.4 L | Flavourzyme | Neutrase | FAO/WHO/UNa | ||||
|---|---|---|---|---|---|---|---|---|
| FMM | HWD | FMM | HWD | FMM | HWD | Child | Adult | |
| Essential Amino acids | ||||||||
| Isoleucine | 3.79 ± 0.08d | 3.48 ± 0.01c | 3.12 ± 0.02a | 3.22 ± 0.11ab | 3.29 ± 0.01b | 3.26 ± 0.01b | 3.0 | 3.0 |
| Leucine | 8.04 ± 0.01ab | 8.23 ± 0.03bc | 8.23 ± 0.03bc | 8.69 ± 0.06d | 7.88 ± 0.12a | 8.26 ± 0.14c | 6.0 | 5.9 |
| Lysine | 9.08 ± 0.01a | 10.14 ± 0.01c | 10.51 ± 0.03d | 10.81 ± 0.06e | 9.87 ± 0.01b | 10.53 ± 0.01d | 4.8 | 4.5 |
| Methionine | 2.96 ± 0.02e | 2.66 ± 0.01c | 2.34 ± 0.01a | 2.52 ± 0.01b | 2.73 ± 0.03d | 2.63 ± 0.01c | 2.3b | 1.6b |
| Met + Cys | 3.60 ± 0.07d | 3.35 ± 0.06c | 2.85 ± 0.01a | 3.11 ± 0.02b | 3.22 ± 0.01c | 3.29 ± 0.02c | ||
| Phenylalanine | 3.78 ± 0.02e | 3.20 ± 0.01d | 3.13 ± 0.01c | 2.99 ± 0.01a | 3.12 ± 0.01bc | 3.09 ± 0.01b | 4.1c | 3.8c |
| Phe + Tyr | 6.70 ± 0.03d | 5.31 ± 0.02c | 4.64 ± 0.01a | 4.66 ± 0.06a | 5.24 ± 0.02bc | 5.16 ± 0.01b | ||
| Threonine | 4.58 ± 0.09d | 4.38 ± 0.04c | 3.95 ± 0.01a | 4.09 ± 0.02b | 4.21 ± 0.05b | 4.39 ± 0.04c | 2.5 | 2.3 |
| Valine | 4.30 ± 0.01f | 4.10 ± 0.01e | 3.77 ± 0.01a | 3.91 ± 0.01b | 3.94 ± 0.01c | 3.98 ± 0.01d | 2.9 | 3.9 |
| Histidine | 2.28 ± 0.06d | 2.09 ± 0.02ab | 2.03 ± 0.03a | 2.09 ± 0.02ab | 2.17 ± 0.06bc | 2.23 ± 0.01cd | 1.6 | 1.5 |
| Tryptophan | 5.42 ± 0.01e | 2.79 ± 0.02d | 1.32 ± 0.01a | 1.67 ± 0.02b | 2.78 ± 0.02d | 2.33 ± 0.02c | 0.66 | 0.6 |
| Nonessential Amino Acid | ||||||||
| Alanine | 6.44 ± 0.02a | 6.81 ± 0.02b | 7.98 ± 0.01f | 7.61 ± 0.01e | 7.06 ± 0.03c | 7.20 ± 0.03d | ||
| Arginine | 5.76 ± 0.02a | 5.97 ± 0.01b | 6.08 ± 0.02c | 6.13 ± 0.04cd | 6.17 ± 0.01d | 6.17 ± 0.01d | ||
| Aspartic acidd | 9.96 ± 0.02c | 10.25 ± 0.01d | 9.91 ± 0.01b | 10.39 ± 0.02e | 9.85 ± 0.02a | 10.59 ± 0.02f | ||
| Cysteinee | 0.66 ± 0.03c | 0.55 ± 0.00b | 0.51 ± 0.01a | 0.56 ± 0.01b | 0.51 ± 0.01a | 0.65 ± 0.02c | ||
| Glutamic acidf | 16.37 ± 0.01a | 18.56 ± 0.01c | 19.62 ± 0.01d | 21.15 ± 0.01e | 18.14 ± 0.01b | 19.61 ± 0.01d | ||
| Glycine | 5.04 ± 0.00c | 4.71 ± 0.01a | 6.68 ± 0.02f | 5.16 ± 0.02d | 5.63 ± 0.01e | 4.90 ± 0.01b | ||
| Serine | 4.09 ± 0.01b | 4.06 ± 0.03b | 3.90 ± 0.01a | 4.07 ± 0.03b | 3.91 ± 0.01a | 4.05 ± 0.02b | ||
| Tyrosine | 2.93 ± 0.04e | 2.17 ± 0.03d | 1.50 ± 0.01a | 1.64 ± 0.01b | 2.12 ± 0.02cfd | 2.08 ± 0.02c | ||
| Proline | 4.42 ± 0.01c | 5.65 ± 0.01e | 5.39 ± 0.01d | 3.26 ± 0.01a | 6.39 ± 0.02f | 3.70 ± 0.03b | ||
| Molecular weight (Da) | Area (%) | |||||
|---|---|---|---|---|---|---|
| Alc FMM | Flav FMM | Neut FMM | Alc HWD | Flav HWD | Neut HWD | |
| >8000 | – | 10.00 | 9.87 | – | 14.32 | 10.96 |
| 3000–8000 | – | 24.71 | 9.87 | 5.68 | 10.17 | 16.52 |
| 2000–3000 | 4.98 | 17.201 | 30.81 | – | – | – |
| 1000–2000 | – | 21.17 | 9.54 | 34.63 | 28.78 | 31.31 |
| 600–1000 | 32.47 | – | 20.04 | – | 13.17 | 21.57 |
| 300–600 | 27.87 | 14.72 | 6.91 | 26.9 | 20.43 | 10.69 |
| 200–300 | 19.54 | – | 5.06 | – | 3.28 | 5.80 |
| < 200 | 15.11 | 12.83 | 7.87 | 32.79 | 9.85 | 3.38 |
| Sample | Antioxidant activity (%) | ||
|---|---|---|---|
| DPPH | ABTS | Fe2+ chelating | |
| FMM | |||
| Alcalase | 86.67 ± 1.15e | 91.27 ± 0.25b | 82.57 ± 0.51d |
| Flavourzyme | 70.20 ± 1.06b | 88.13 ± 0.23a | 75.80 ± 0.72ab |
| Neutrase | 82.00 ± 1.73cd | 93.33 ± 0.58c | 77.23 ± 0.32bc |
| HWD | |||
| Alcalase | 84.67 ± 0.58de | 92.60 ± 1.30bc | 78.00 ± 0.20c |
| Flavourzyme | 64.67 ± 0.58a | 93.50 ± 0.71c | 75.00 ± 1.00a |
| Neutrase | 79.67 ± 1.53c | 94.23 ± 0.68c | 78.32 ± 0.40c |
| Sample | FMM | HWD | ||||
|---|---|---|---|---|---|---|
| Alc. | Flav. | Neut. | Alc. | Flav. | Neut. | |
| IVPD (%) | 92.73 ± 0.76b | 88.23 ± 0.06a | 92.43 ± 0.06b | 93.2 ± 0.20b | 89.37 ± 0.67a | 92.83 ± 0.76b |
| WHC (mL/g) | 2.10 ± 0.10a | 2.77 ± 0.06b | 2.57 ± 0.12b | 1.77 ± 0.06a | 2.10 ± 0.17a | 2.57 ± 0.06b |
| OHC (mL/g) | 2.27 ± 0.06a | 3.07 ± 0.06c | 2.77 ± 0.06bc | 2.23 ± 0.25a | 2.57 ± 0.06ab | 3.13 ± 0.15c |
| EC (mL/0.5g) | 22.33 ± 0.58ab | 27.33 ± 0.58c | 22.50 ± 0.10ab | 21.40 ± 0.36a | 26.40 ± 0.17c | 23.20 ± 0.20b |
| BD (g/mL) | 0.45 ± 0.01ab | 0.35 ± 0.01a | 0.53 ± 0.06b | 0.53 ± 0.06b | 0.34 ± 0.01a | 0.46 ± 0.01ab |
| FC (g/mL) | 125.50 ± 0.10de | 138.50 ± 0.50b | 130.20 ± 0.76c | 124.50 ± 1.08e | 137.50 ± 0.20a | 129.60 ± 0.58d |
| Enzyme | Form | pH | T (°C) |
|---|---|---|---|
| Alcalase 2.4 L(AU/g)* | Liquid/grain | 8.0 | 55 |
| Flavourzyme (500 LAPU/g)† | Powder | 7.0 | 50 |
| Neutrase (1.5 AU/g) | Liquid/grain | 7.0 | 45 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Foh, M.B.K.; Amadou, I.; Foh, B.M.; Kamara, M.T.; Xia, W. Functionality and Antioxidant Properties of Tilapia (Oreochromis niloticus) as Influenced by the Degree of Hydrolysis. Int. J. Mol. Sci. 2010, 11, 1851-1869. https://doi.org/10.3390/ijms11041851
Foh MBK, Amadou I, Foh BM, Kamara MT, Xia W. Functionality and Antioxidant Properties of Tilapia (Oreochromis niloticus) as Influenced by the Degree of Hydrolysis. International Journal of Molecular Sciences. 2010; 11(4):1851-1869. https://doi.org/10.3390/ijms11041851
Chicago/Turabian StyleFoh, Mohamed Beva Kelfala, Issoufou Amadou, Betty Mabel Foh, Mohamed Tabita Kamara, and Wenshui Xia. 2010. "Functionality and Antioxidant Properties of Tilapia (Oreochromis niloticus) as Influenced by the Degree of Hydrolysis" International Journal of Molecular Sciences 11, no. 4: 1851-1869. https://doi.org/10.3390/ijms11041851




