Biological Properties of Iron Oxide Nanoparticles for Cellular and Molecular Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of Particle Internalization and Internalization Kinetics in Vitro
2.2. Long-Term Stability of Labeling in Vitro
2.3. Cellular Toxicity and Plasma Binding
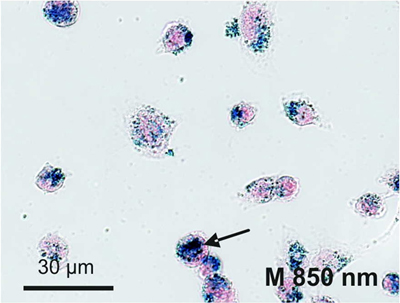
2.4. Cellular Localization of Absorbed Iron Oxide Nanoparticles
2.5. MRI Visualization in Vitro
3. Experimental Section
3.1. Cell Culture, Contrast Agents and Cell Labeling
3.2. Quantification of Iron
3.3. Cell-Toxicity
3.4. Visualization by Prussian Blue Staining and Electron Microscopy
3.5. Magnetic Resonance Imaging (MRI)
4. Conclusions
Acknowledgements
References
- Hahn, MA; Singh, AK; Sharma, P; Brown, SC; Moudgil, BM. Nanoparticles as contrast agents for in vivo bioimaging: current status and future perspectives. Anal Bioanal Chem 2010, 4207–5. [Google Scholar] [CrossRef]
- Grimm, J; Kircher, M; Weissleder, R. Cell tracking: Principles and applications. Radiologe 2007, 47, 25–33. [Google Scholar]
- Yoo, BM; Pagel, D. An overview of responsive MRI contrast agents for molecular imaging. Front. Biosci 2008, 13, 1733–1752. [Google Scholar]
- Nunn, AD; Linder, KE; Tweedle, MF. Can receptors be imaged with MRI agents? Q. J. Nucl. Med 1997, 41, 155–162. [Google Scholar]
- Moore, A; Weissleder, R; Bogdanov, A, Jr. Uptake of dextran-coated monocrystalline iron oxides in tumor cells macrophages. J. Magn. Reson Imaging 1997, 7, 1140–1145. [Google Scholar]
- Muldoon, L; Sàndor, M; Pinkston, K; Neuwelt, E. Imaging, distribution and toxicity of superparamagnetic iron oxide magnetic resonance agents in rat brain and intracerebral tumor. Neurosurgery 2005, 57, 785–796. [Google Scholar]
- Heneweer, C; Kossel, E; Schlorf, T; Both, M; Wolter, J; Mentlein, R; Glueer, CC; Heller, M. Artefact-free imaging of iron oxide labeled monolayers with magnetic resonance at 3T. Proceedings of Molekulare Bildgebung 2006 Conference, Jena, Germany; 2006. [Google Scholar]
- Mentlein, R; Held-Feindt, J. Pleiotrophin, an angiogenic and mitogenic growth factor, is expressed in human gliomas. J. Neurochem 2002, 83, 747–753. [Google Scholar]
- Ludwig, A; Schulte, A; Schnack, C; Hundhausen, C; Reiss, K; Brodway, N; Held-Feindt, J; Mentlein, R. Enhanced expression and shedding of the transmembrane chemokine CXCL16 by reactive astrocytes and glioma cells. J. Neurochem 2005, 93, 1293–1303. [Google Scholar]
- Mentlein, R; Buchholz, C; Krisch, B. Binding and internalization of gold-conjugated somatostatin and growth hormone-releasing hormone in cultured rat somatotropes. Cell Tissue Res 1989, 258, 309–317. [Google Scholar]
- Meincke, M; Schlorf, T; Kossel, E; Jansen, O; Glueer, CC; Mentlein, R. Iron oxide-loaded liposomes for MR imaging. Front. Biosci 2008, 13, 4002–4008. [Google Scholar]
- Thorek, DL; Tsourkas, A. Size, charge and concentration dependent uptake of iron oxide particles by non-phagocytic cells. Biomaterials 2008, 29, 3583–3590. [Google Scholar]
- Zhang, Z; van den Bos, EJ; Wielopolski, PA; de Jong-Popijus, M; Bernsen, MR; Duncker, DJ; Krestin, GP. In vitro imaging of single living human umbilical vein endothelial cells with a clinical 3.0-T MRI scanner. MAGMA 2005, 18, 175–185. [Google Scholar]
- Ittrich, H; Lange, C; Dahnke, H; Zander, AR; Adam, G; Nolte-Ernsting, C. Labeling of mesenchymal stem cells with different superparamagnetic particles of iron oxide and detectability with MRI at 3T. RöFo 2005, 177, 1151–1163. [Google Scholar]
- Ittrich, H; Lange, C; Tögel, F; Zander, AR; Dahnke, H; Westenfelder, C; Adam, G; Nolte-Ernsting, C. In vivo magnetic resonance imaging of iron oxide-labeled, arterially-injected mesenchymal stem cells in kidneys of rats with acute ischemic kidney injury: detection and monitoring at 3T. J. Magn. Reson Imaging 2007, 25, 1179–1191. [Google Scholar]
- Lemarchand, C; Gref, R; Passirani, C; Garcion, E; Petri, B; Müller, R; Costantini, D; Couvreur, P. Influence of polysaccharide coating on the interactions of nanoparticles with biological systems. Biomaterials 2006, 27, 108–118. [Google Scholar]






| Particle | Core | Coating | Hydrodynamic Diameter | Supplier |
|---|---|---|---|---|
| Resovist | Maghemite/magnetite | Carboxydextran | 60 nm | Schering (Berlin, Germany) |
| B102 | Maghemite/magnetite | Carboxymethyldextran | 60 nm | Dr. Norbert Buske (Berlin, Germany) |
| Non-coated Fe3O4 | Magnetite | None | 10 nm | Dr. Norbert Buske (Berlin, Germany) |
| Microspheres (M310) | 30–60% Ferrite | Polystyrene | 310 nm | Merck Estapor (Fontenay Sous Bois, France) |
| Microspheres (M850) | 55–65% Ferrite | Polystyrene | 850 nm | Merck Estapor (Fontenay Sous Bois, France) |
| Nanomag20 (N20) | Magnetite | Dextran | 20 nm | Micromod (Rostock, Germany) |
| Nanomag70 (N70) | Maghemite | Dextran | 70 nm | Micromod (Rostock, Germany) |
| Nanomag100 (N100) | Maghemite | Dextran | 100 nm | Micromod (Rostock, Germany) |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schlorf, T.; Meincke, M.; Kossel, E.; Glüer, C.-C.; Jansen, O.; Mentlein, R. Biological Properties of Iron Oxide Nanoparticles for Cellular and Molecular Magnetic Resonance Imaging. Int. J. Mol. Sci. 2011, 12, 12-23. https://doi.org/10.3390/ijms12010012
Schlorf T, Meincke M, Kossel E, Glüer C-C, Jansen O, Mentlein R. Biological Properties of Iron Oxide Nanoparticles for Cellular and Molecular Magnetic Resonance Imaging. International Journal of Molecular Sciences. 2011; 12(1):12-23. https://doi.org/10.3390/ijms12010012
Chicago/Turabian StyleSchlorf, Thomas, Manuela Meincke, Elke Kossel, Claus-Christian Glüer, Olav Jansen, and Rolf Mentlein. 2011. "Biological Properties of Iron Oxide Nanoparticles for Cellular and Molecular Magnetic Resonance Imaging" International Journal of Molecular Sciences 12, no. 1: 12-23. https://doi.org/10.3390/ijms12010012