Environmental Dependence of Artifact CD Peaks of Chiral Schiff Base 3d-4f Complexes in Soft Mater PMMA Matrix
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure of NdNi
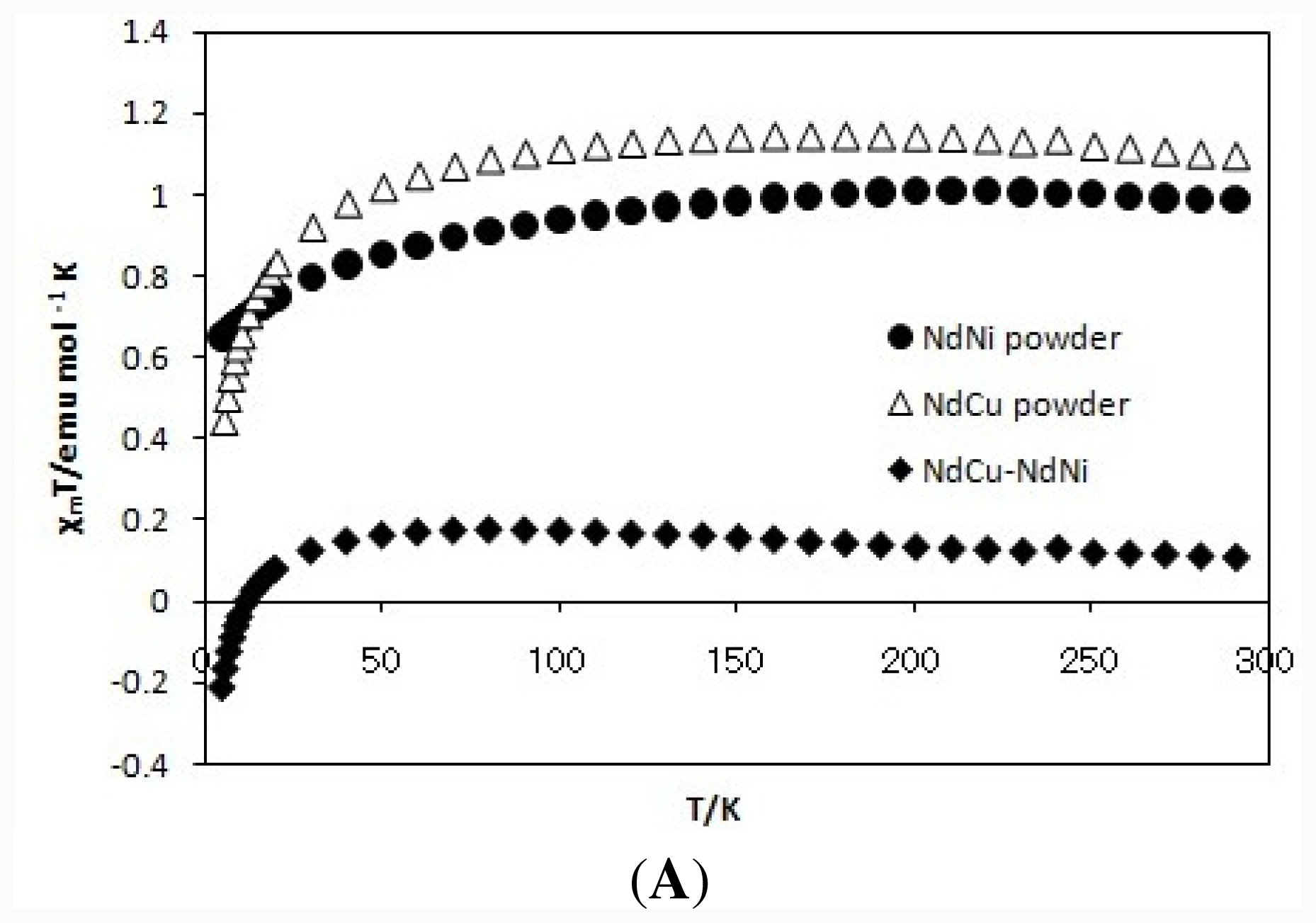
2.2. Magnetic Properties
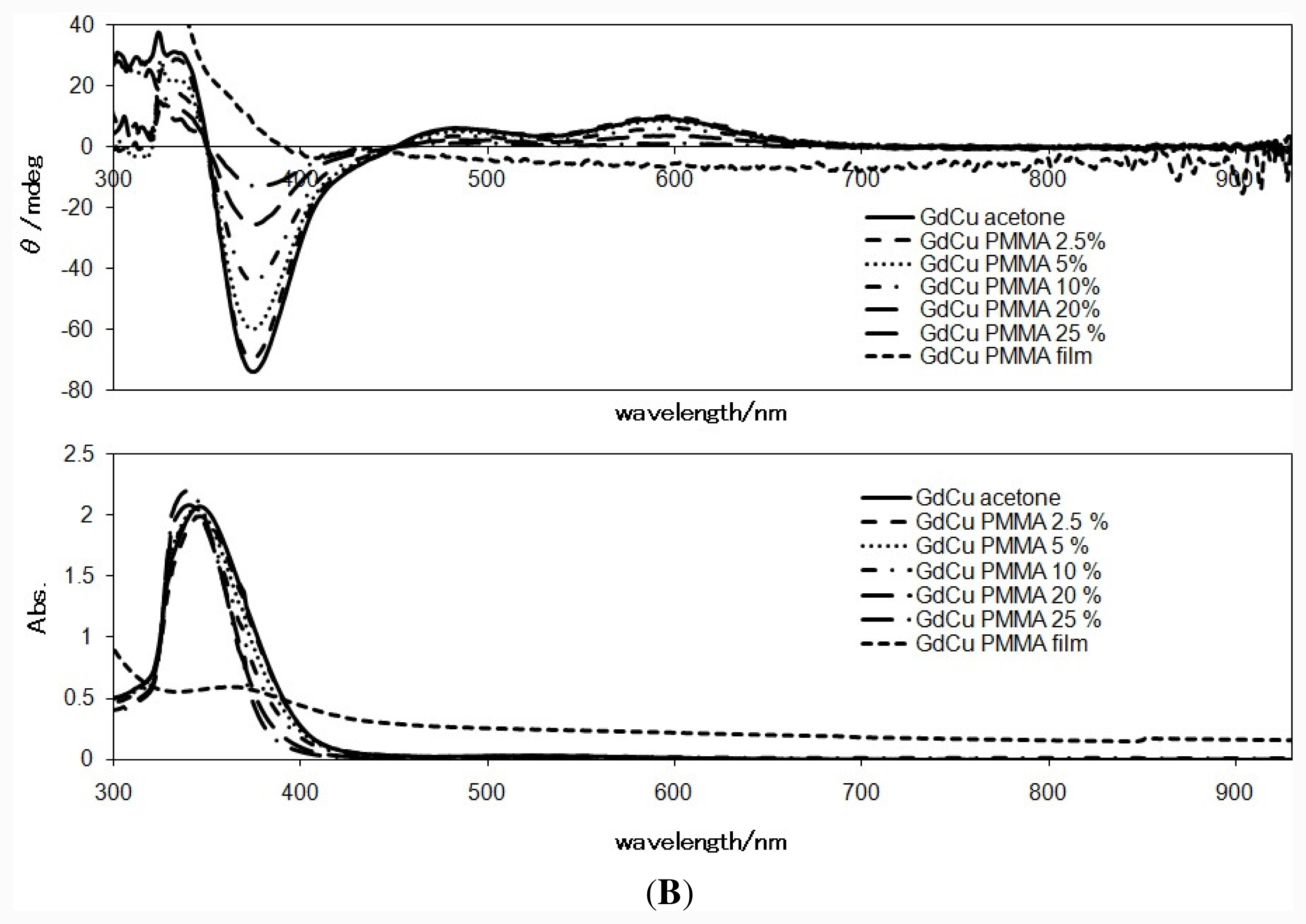
2.3. CD Spectra in the Solid State
2.4. CD Spectra in PMMA Films or Solutions
3. Experimental Section
3.1. General Procedures
3.2. Preparations
3.2.1. Preparation of NdNi
3.2.2. Preparation of NdCu
3.2.3. Preparation of GdNi
3.2.4. Preparation of GdCu
3.3. Physical Measurements
3.4. X-ray Crystallography
4. Conclusions
Supplementary Data
References
- Akitsu, T; Einaga, Y. Syntheses, crystal structures, and electronic properties of a series of copper(II) complexes with 3,5-halogen-substituted Schiff base ligands and their solutions. Polyhedron 2005, 24, 2933–2943. [Google Scholar]
- Akitsu, T; Einaga, Y. Synthesis, crystal structures, and electronic properties of Schiff base nickel(II) complexes: towards solvatochromism induced by photochromic solute. Polyhedron 2005, 24, 1869–1877. [Google Scholar]
- Akitsu, T. Photofunctional supramolecular solution systems of chiral Schiff base nickel(II), copper(II), and zinc(II) complexes and photochromic azobenzenes. Polyhedron 2007, 26, 2527–2535. [Google Scholar]
- Akitsu, T; Einaga, Y. Novel photo-induced aggregation behavior of a supramolecular system containing iron(III) magnetic ionic liquid and azobenzene. Inorg. Chem. Commun 2006, 9, 1108–1110. [Google Scholar]
- Akitsu, T; Nishijo, J. The first observation of photoinduced tuning of AC magnetization for organic/inorganic hybrid materials composed of Mn12-acetate and azobenzene. J. Magn. Magn. Mater 2007, 315, 95–100. [Google Scholar]
- Akitsu, T; Nishijo, J. The first detection of photomodulation by both DC and in-phase AC susceptibility for organic/inorganic hybrid materials containing cyano-bridged Gd-Cr complex and azobenzene. J. Magn. Magn. Mater 2008, 320, 1586–1590. [Google Scholar]
- Akitsu, T. The tuning of quantum magnetization for organic/inorganic hybrid materials composed of Mn12-acetate and azobenzene casted into PMMA films on PVA films. J. Magn. Magn. Mater 2008, 321, 207–212. [Google Scholar]
- Akitsu, T; Ishioka, C; Itoh, T. Polarized Spectroscopy of Hybrid Materials of Mn12 Single-Molecule Magnet and Azobenzene or Disperse Red 1 in PMMA Films. Cent. Eur. J. Chem 2009, 7, 690–696. [Google Scholar]
- Akitsu, T; Itoh, T. Polarized spectroscopy of hybrid materials of chiral Schiff base cobalt(II), nickel(II), copper(II), and zinc(II) complexes and photochromic azobenzenes in PMMA films. Polyhedron 2010, 29, 477–487. [Google Scholar]
- Akitsu, T; Ishioka, C. Manipulation and Observation by Polarized Light: Hybrid Materials of Chiral Schiff Base Mn(III) Complexes and Azobenzene in PMMA. Asian Chem. Lett 2010, 14, 37–51. [Google Scholar]
- Aritake, Y; Takanashi, T; Yamazaki, A; Akitsu, T. Polarized spectroscopy and hybrid materials of chiral Schiff base Ni(II), Cu(II), Zn(II) complexes with included or separated azo-groups. Polyhedron 2011, 30, 886–894. [Google Scholar]
- Akitsu, T; Miura, Y. Polarized electronic spectra of organic/inorganic hybrid materials of chiral Schiff base Ni(II) or Cu(II) complexes and disperse red 1 or azobenzene in PMMA films. J. Chem. Chem. Eng 2011, 5, 443–450. [Google Scholar]
- Akitsu, T; Tanaka, R. Polarized Electronic and IR Spectroscopy of Hybrid Materials of Chiral Cu(II) and Mn12 Complexes and Some Photochromic Compounds in PMMA Films. Asian Chem. Lett 2010, 14, 235–254. [Google Scholar]
- Akitsu, T; Tanaka, R. Polarized Electronic and IR Spectra of Hybrid Materials of Chiral Mn(II) Complexes and Different Types of Photochromic Dyes Showing Photoisomerization or Weigert Effect. Curr. Phys. Chem 2011, 1, 82–89. [Google Scholar]
- Akitsu, T; Yoshida, A. Detection of Intermolecular Interaction Between Pd(II) Complexes Having Imidate and Amine Ligands and Photochromic Dyes in Hybrid Solution Systems by Absorption and Fluorescence Spectra. Curr. Phys. Chem 2011, 1, 76–81. [Google Scholar]
- Kuroda, R; Harada, T; Shindo, Y. A solid-strate dedicated circular dichroism spectrophotometer: Development and application. Rev. Sci. Instr 2001, 72, 3802–3810. [Google Scholar]
- Schellaman, J; Jensen, HP. Optical spectroscopy of oriented molecules. Chem. Rev 1987, 87, 1359–1399. [Google Scholar]
- Tinoco, I; Mickols, W; Maestre, MF; Bustamante, C. Absorption, scattering, and imaging of biomolecular structures with polarized light. Annu. Rev. Biophys. Biophys. Chem 1987, 16, 319–349. [Google Scholar]
- Claborn, K; Isborn, C; Kaminsky, W; Kahr, B. Optical rotation of achiral comounds. Angew. Chem. Int. Ed. Engl 2008, 47, 5706–5717. [Google Scholar]
- Kaminsky, W. Experimental and phenomenological aspects of circular birefringence and related properties in transparent crystals. Rep. Prog. Phys 2000, 63, 1575–1640. [Google Scholar]
- Shindo, Y; Nakagawa, M. On the artifacts in circularly polarized emission spectroscopy. Appl. Spectrosc 1985, 39, 32–38. [Google Scholar]
- Shindo, Y; Nakagawa, M; Ohmi, T. On the problems of CD spectropolarimeters.II: artifacts in CD spectrometers. Appl. Spectrosc 1985, 39, 860–868. [Google Scholar]
- Hipps, KW; Crosby, GA. Applications of the photoelastic modulator to polarization spectroscopy. J. Phys. Chem 1979, 83, 555–562. [Google Scholar]
- Goldstein, DH. Polarized Light; Marcel Drekker: New York, NY, USA, 2003. [Google Scholar]
- Kobayashi, J; Uesu, Y; Takehara, H. A new optical method and apparatus “HAUP” for measuring simultaneously optical activity and birefringence of crystals. II. Application to triglycine-sulphuric acid (NH2CH2CO2H)3.H2SO4. J. Appl. Crystallogr 1983, 16, 212–219. [Google Scholar]
- Kaminsky, W; Claborn, K; Kahr, B. Polarimetric imaging of crystals. Chem. Soc. Rev 2004, 33, 514–525. [Google Scholar]
- Kahr, B; Bing, Y; Kaminsky, W; Viterbo, D. Turinese stereochemistry: Eligio Perucca’s enantioselectivity and Primo Levi’s asymmetry. Angew. Chem. Int. Ed 2009, 48, 3744–3748. [Google Scholar]
- Bing, Y; Selassie, D; Paradise, RH; Isborn, C; Kramer, N; Sadilek, M; Kaminsky, W; Kahr, B. Circular dichroism tensor of a triarylmethyl propeller in sodium chlorate. J. Am. Chem. Soc 2010, 132, 7454–7465. [Google Scholar]
- Kaminsky, W; Geday, MA; Herreros-Cedrés, J; Kahr, B. Optical rotatory and circular dichroic scattering. J. Phys. Chem. A 2003, 107, 2800–2807. [Google Scholar]
- Kaminsky, W; Herreros-Cedrés, J; Geday, MA; Kahr, B. Dispersion of anomalous azimuthal rotation and circular extinction contrast in dyed K2SO4 crystals. Chirality 2004, 16, S55–S61. [Google Scholar]
- Claborn, K; Chu, AS; Jang, SH; Su, FY; Kaminsky, W; Kahr, B. Circular extinction imaging: determination of the absolute orientation of embedded chromophores in enantiomorphously twinned LiKSO4 crystals. Cryst. Growth Des 2005, 5, 2117–2123. [Google Scholar]
- Hazra, S; Koner, R; Nayak, M; Sparkes, HA; Howard, JAK; Mohanta, S. Cocrystallized dinuclear-mononuclear CuII3NaI and double-decker-triple-decker CuII5KI3 complexes derived from N,N’-Ethylenebis(3-ethoxysalicylaldimine). Cryst. Growth Des 2009, 9, 3603–3608. [Google Scholar]
- Akine, S; Taniguchi, T; Nabeshima, T. Cooperative formation of trinuclear Zinc(II) complexes via complexation of a tetradentate oxime chelate ligand, salamo, and Zinc(II) acetate. Inorg. Chem 2004, 43, 6142–6144. [Google Scholar]
- Jana, A; Majumder, S; Carrella, L; Nayak, M; Weyhermueller, T; Dutta, S; Schollmeyer, D; Rentschler, E; Koner, R; Mohanta, S. Syntheses, structures, and magnetic properties of diphenoxo-bridged CuIILnIII and NiII(Low-Spin)LnIII compounds derived from a compartmental ligand (Ln = Ce-Yb). Inorg. Chem 2010, 49, 9012–9025. [Google Scholar]
- Andruh, M; Costes, JP; Diaz, C; Gao, S. 3d-4f combined chemistry: synthetic strategies and magnetic properties. Inorg. Chem 2009, 48, 3342–3359. [Google Scholar]
- Burrow, CE; Burchell, TJ; Lin, PH; Habib, F; Wernsdorfer, W; Clerac, R; Murugesu, M. Salen-Based [Zn2Ln3] complexes with fluorescence and single-molecule-magnet properties. Inorg. Chem 2009, 48, 8051–8053. [Google Scholar]
- Yang, XP; Jones, RA; Wong, WK; Lynch, V; Oye, MM; Holmes, AL. Design and synthesis of a near infra-red luminescent hexanuclear Zn-Nd prism. Chem Commun 2006, 1836–1838. [Google Scholar]
- Wong, WK; Yang, X; Jones, RA; Rivers, JH; Lynch, V; Lo, WK; Xiao, D; Oye, MM; Holmes, AL. Multinuclear luminescent schiff-base Zn-Nd sandwich complexes. Inorg. Chem 2006, 45, 4340–4345. [Google Scholar]
- Pasatoiu, TD; Madalan, AM; Kumke, MU; Tiseanu, C; Andruh, M. Temperature switch of lmct role: from quenching to sensitization of europium emission in a ZnII-EuIII binuclear complex. Inorg. Chem 2010, 49, 2310–2315. [Google Scholar]
- Sheldrick, GM. A short history of SHELX. Acta Cryst. A 2007, 64, 112–122. [Google Scholar]

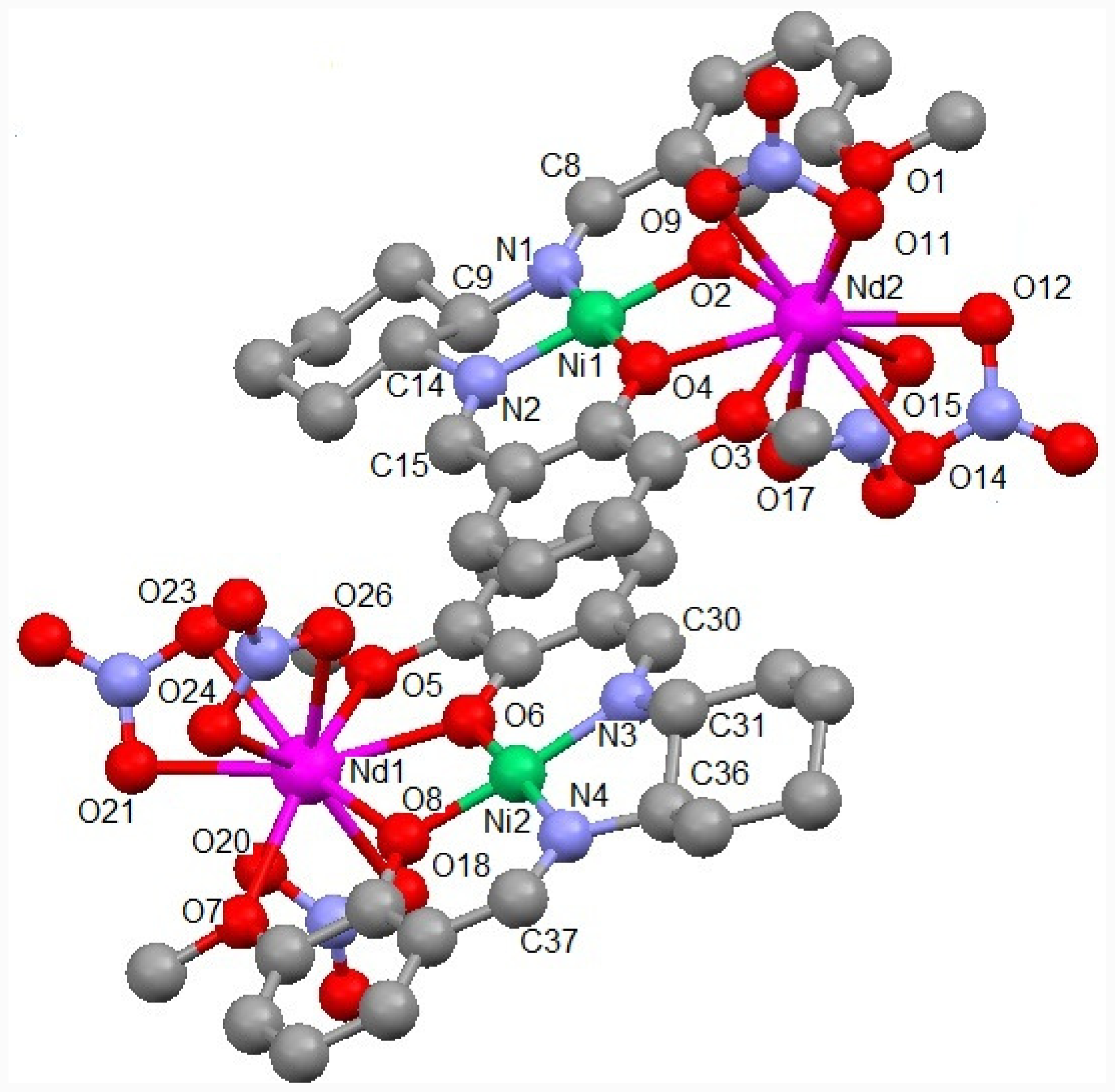




| Nd1-O6 = 2.374(7) | Nd1-O8 = 2.377(6) | Nd1-O20 = 2.468(8) | Nd1-O18 = 2.470(7) |
| Nd1-O23 = 2.477(7) | Nd1-O21 = 2.482(7) | Nd1-O26 = 2.510(7) | Nd1-O24 = 2.514(7) |
| Nd1-O5 = 2.528(6) | Nd1-O7 = 2.543(6) | Nd1-N8 = 2.873(8) | Nd1-N9 = 2.902(8) |
| Nd2-O2 = 2.337(7) | Nd2-O4 = 2.395(7) | Nd2- O11 = 2.463(9) | Nd2-O9 = 2.478(7) |
| Nd2-O15 = 2.504(7) | Nd2-O1 = 2.507(6) | Nd2-O14 = 2.518(6) | Nd2-O3 = 2.522(7) |
| Nd2-O12 = 2.523(7) | Nd2-O17 = 2.533(9) | Nd2-N5 = 2.921(11) | Nd2-N7 = 2.942(9) |
| Ni1-O4 = 1.838(6) | Ni1-N2 = 1.843(7) | Ni1-N1 = 1.844(8) | Ni1-O2 = 1.876(7) |
| Ni2-O8 = 1.833(6) | Ni2-N4 = 1.849(8) | Ni2-N3 = 1.855(8) | Ni2-O6 = 1.861(7) |
| O4-Ni1-N2 = 94.3(3) | O4-Ni1-N1 = 175.9(3) | N2-Ni1-N1 = 88.7(3) | O4-Ni1-O2 = 81.9(3) |
| N2-Ni1-O2 = 175.8(3) | N1-Ni1-O2 = 95.2(3) | O8-Ni2-N4 = 95.3(3) | O8-Ni2-N3 = 175.7(3) |
| N4-Ni2-N3 = 85.9(3) | O8-Ni2-O6 = 82.8(3) | N4-Ni2-O6 = 175.3(3) | N3-Ni2-O6 = 96.3(3) |
| O6-Nd1-O8 = 61.9(2) | O6-Nd1-O20 = 117.0(3) | O8-Nd1-O20 = 118.6(2) | O6-Nd1-O18 = 73.6(3) |
| O8-Nd1-O18 = 74.9(2) | O20-Nd1-O18 = 51.4(3) | O6-Nd1-O23 = 110.7(2) | O8-Nd1-O23 = 144.0(2) |
| O20-Nd1-O23 = 96.5(2) | O18-Nd1-O23 = 139.6(2) | O6-Nd1-O21 = 161.2(2) | O8-Nd1-O21 = 134.3(2) |
| O20-Nd1-O21 = 66.8(3) | O18-Nd1-O21 = 116.6(3) | O23-Nd1-O21 = 51.0(2) | O6-Nd1-O26 = 68.1(2) |
| O8-Nd1-O26 = 77.1(2) | O20-Nd1-O26 = 164.3(2) | O18-Nd1-O26 = 139.9(2) | O23-Nd1-O26 = 68.1(2) |
| O21-Nd1-O26 = 103.5(2) | O6-Nd1-O24 = 111.9(2) | O8-Nd1-O24 = 77.6(2) | O20-Nd1-O24 = 130.3(2) |
| O18-Nd1-O24 = 144.6(2) | O23-Nd1-O24 = 73.3(2) | O21-Nd1-O24 = 69.8(3) | O26-Nd1-O24 = 50.2(2) |
| O6-Nd1-O5 = 63.2(2) | O8-Nd1-O5 = 124.0(2) | O20-Nd1-O5 = 78.2(2) | O18-Nd1-O5 = 80.4(2) |
| O23-Nd1-O5 = 68.0(2) | O21-Nd1-O5 = 101.7(2) | O26-Nd1-O5 = 92.4(2) | O24-Nd1-O5 = 134.3(2) |
| O6-Nd1-O7 = 123.8(2) | O8-Nd1-O7 = 64.4(2) | O20-Nd1-O7 = 76.1(2) | O18-Nd1-O7 = 77.9(2) |
| O23-Nd1-O7 = 122.2(2) | O21-Nd1-O7 = 74.8(2) | O26-Nd1-O7 = 114.3(2) | O24-Nd1-O7 = 70.2(2) |
| O5-Nd1-O7 = 153.2(2) | O6-Nd1-N8 = 97.3(2) | O8-Nd1-N8 = 95.4(2) | O20-Nd1-N8 = 25.7(2) |
| O18-Nd1-N8 = 26.1(3) | O23-Nd1-N8 = 120.5(2) | O21-Nd1-N8 = 90.8(2) | O26-Nd1-N8 = 165.4(2) |
| O24-Nd1-N8 = 141.1(2) | O5-Nd1-N8 = 81.3(2) | O7-Nd1-N8 = 72.3(2) | O6-Nd1-N9 = 137.1(2) |
| O8-Nd1-N9 = 145.1(2) | O20-Nd1-N9 = 81.6(2) | O18-Nd1-N9 = 132.8(3) | O23-Nd1-N9 = 26.4(2) |
| O21-Nd1-N9 = 24.6(2) | O26-Nd1-N9 = 85.2(2) | O24-Nd1-N9 = 68.0(2) | O5-Nd1-N9 = 86.1(2) |
| O7-Nd1-N9 = 97.3(2) | N8-Nd1-N9 = 107.3(2) | O2-Nd2-O4 = 61.9(2) | O2-Nd2-O11 = 119.4(3) |
| O4-Nd2-O11 = 118.3(3) | O2-Nd2-O9 = 76.9(2) | O4-Nd2-O9 = 72.9(3) | O11-Nd2-O9 = 52.0(3) |
| O2-Nd2-O15 = 79.1(2) | O4-Nd2-O15 = 114.1(2) | O11-Nd2-O15 = 127.0(3) | O9-Nd2-O15 = 147.3(2) |
| O2-Nd2-O1 = 62.8(2) | O4-Nd2-O1 = 121.8(2) | O11-Nd2-O1 = 75.8(3) | O9-Nd2-O1 = 78.6(2) |
| O15-Nd2-O1 = 70.7(2) | O2-Nd2-O14 = 141.3(3) | O4-Nd2-O14 = 111.1(2) | O11-Nd2-O14 = 97.8(3) |
| O9-Nd2-O14 = 140.3(2) | O15-Nd2-O14 = 69.5(2) | O1-Nd2-O14 = 122.9(2) | O2-Nd2-O3 = 123.2(2) |
| O4-Nd2-O3 = 61.9(2) | O11-Nd2-O3 = 81.3(3) | O9-Nd2-O3 = 79.7(3) | O15-Nd2-O3 = 132.5(2) |
| O1-Nd2-O3 = 155.2(2) | O14-Nd2-O3 = 69.5(2) | O2-Nd2-O12 = 134.9(2) | O4-Nd2-O12 = 160.91(19) |
| O11-Nd2-O12 = 65.1(3) | O9-Nd2-O12 = 116.2(3) | O15-Nd2-O12 = 68.2(3) | O1-Nd2-O12 = 77.2(2) |
| O14-Nd2-O12 = 50.7(2) | O3-Nd2-O12 = 101.9(2) | O2-Nd2-O17 = 74.6(3) | O4-Nd2-O17 = 67.3(3) |
| O11-Nd2-O17 = 166.0(3) | O9-Nd2-O17 = 138.5(3) | O15-Nd2-O17 = 51.6(2) | O1-Nd2-O17 = 113.1(3) |
| O14-Nd2-O17 = 68.3(3) | O3-Nd2-O17 = 91.3(3) | O12-Nd2-O17 = 105.3(2) | O2-Nd2-N5 = 96.9(3) |
| O4-Nd2-N5 = 95.2(3) | O11-Nd2-N5 = 27.2(3) | O9-Nd2-N5 = 24.8(3) | O15-Nd2-N5 = 143.0(3) |
| O1-Nd2-N5 = 74.8(3) | O14-Nd2-N5 = 121.8(3) | O3-Nd2-N5 = 80.5(3) | O12-Nd2-N5 = 91.6(3) |
| O17-Nd2-N5 = 162.5(3) | O2-Nd2-N7 = 76.9(2) | O4-Nd2-N7 = 92.1(3) | O11-Nd2-N7 = 149.4(3) |
| O9-Nd2-N7 = 153.6(2) | O15-Nd2-N7 = 25.0(3) | O1-Nd2-N7 = 92.0(3) | O14-Nd2-N7 = 65.1(2) |
| O3-Nd2-N7 = 112.7(2) | O12-Nd2-N7 = 85.0(2) | O17-Nd2-N7 = 26.7(3) |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Okamoto, Y.; Nidaira, K.; Akitsu, T. Environmental Dependence of Artifact CD Peaks of Chiral Schiff Base 3d-4f Complexes in Soft Mater PMMA Matrix. Int. J. Mol. Sci. 2011, 12, 6966-6979. https://doi.org/10.3390/ijms12106966
Okamoto Y, Nidaira K, Akitsu T. Environmental Dependence of Artifact CD Peaks of Chiral Schiff Base 3d-4f Complexes in Soft Mater PMMA Matrix. International Journal of Molecular Sciences. 2011; 12(10):6966-6979. https://doi.org/10.3390/ijms12106966
Chicago/Turabian StyleOkamoto, Yu, Keisuke Nidaira, and Takashiro Akitsu. 2011. "Environmental Dependence of Artifact CD Peaks of Chiral Schiff Base 3d-4f Complexes in Soft Mater PMMA Matrix" International Journal of Molecular Sciences 12, no. 10: 6966-6979. https://doi.org/10.3390/ijms12106966




