A Sandwich Electrochemical Immunosensor Using Magnetic DNA Nanoprobes for Carcinoembryonic Antigen
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Different NP Complexes
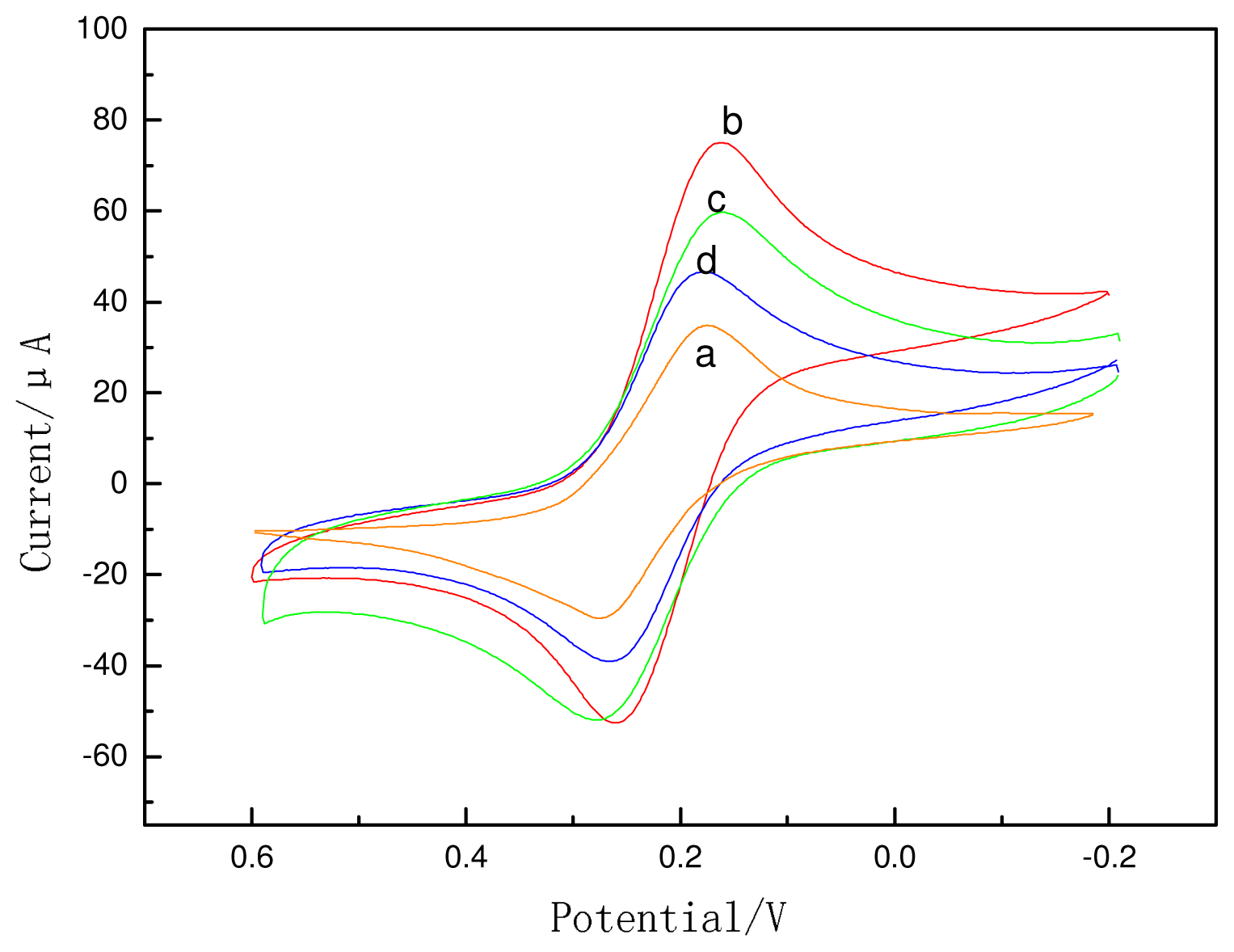
2.2. Electrochemical Behaviors of the ECI
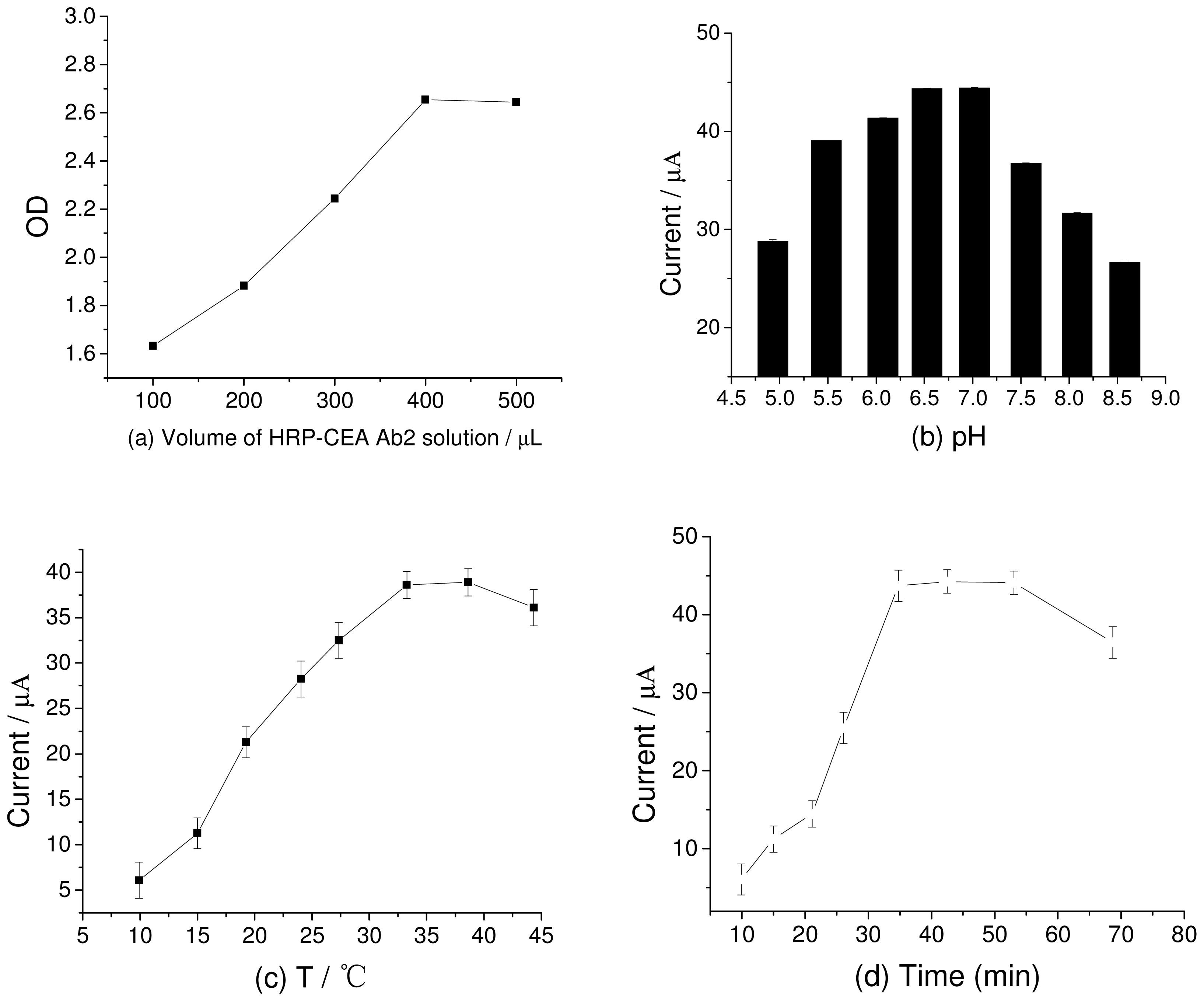
2.3. Optimization of Experimental Conditions
2.4. Detection of CEA
2.5. Precision, Reproducibility, and Stability
2.6. Application of the Immunosensor for Detecting CEA in Human Serum
3. Experimental Section
3.1. Reagents and Chemicals
3.2. Apparatus
3.3. Synthesis of Magnetic Cross-Liked Nanochain Probes
3.4. Designing of the Immunosensor Immunoassay Procedure for CEA Detection
3.5. Immunoassay Procedure for CEA Detection
4. Conclusions
Acknowledgments
References
- Thomas, S.N.; Tong, Z.Q.; Stebe, K.J.; Konstantopoulos, K. Identification, characterization and utilization of tumor cell selectin ligands in the design of colon cancer diagnostics. Biorheology 2009, 46, 207–225. [Google Scholar]
- Konstantopoulos, K.; Thomas, S.N. Cancer cells in transit: The vascular interactions of tumor cells. Annu. Rev. Biomed. Eng 2009, 11, 177–202. [Google Scholar]
- Tang, D.; Yuan, R.; Chai, Y. Magnetic core-shell Fe3O4@Ag nanoparticle-coated carbon paste interface for studies of carcinoembryonic antigen in clinical immunoassay. J. Phys. Chem. B 2006, 110, 11640–11646. [Google Scholar]
- Ci, Y.-X.; Qin, Y.; Chang, W.-B.; Li, Y.-Z. Application of a mimetic enzyme for the enzyme immunoassay for [alpha]-1-fetoprotein. Anal. Chim. Acta 1995, 300, 273–276. [Google Scholar]
- Lianidou, E.S.; Ioannoutichia, P.C.; Sacharidou, E. Second derivative synchronous scanning fluorescence spectrometry as a sensitive detection technique in immunoassays. Application to the determination of [alpha]-fetoprotein. Anal. Chim. Acta 1994, 290, 159–165. [Google Scholar]
- Du, D.; Wang, L.; Shao, Y.; Wang, J.; Engelhard, M.H.; Lin, Y. Functionalized grapheme oxide as a nanocarrier in a mulienzyme labeling amplification strategy for ultrasensitive electrochemical immunoassay of phosphorylated p53(S392). Anal. Chem 2011, 83, 746–752. [Google Scholar]
- Wei, Q.; Xiang, Z.; He, J.; Wang, G.; Li, H.; Qian, Z.; Yang, M. Dumbbell-like Au-Fe3O4 nanoparticles as label for the preparation of electrochemical immunosensors. Biosens. Bioelectron 2010, 26, 627–631. [Google Scholar]
- Ran, X.Q.; Yuan, R.; Chai, Y.Q.; Hong, C.L.; Qian, X.Q. A sensitive amperometric immunosensor for alpha-fetoprotein based on carbon nanotube/DNA/Thi/nano-Au modified glassy carbon electrode. Colloids Surf. B: Biointerfaces 2010, 79, 421–426. [Google Scholar]
- Du, D.; Zou, Z.; Shin, Y.; Wang, J.; Wu, H.; Engelhard, M.H.; Liu, J.; Aksay, I.A.; Lin, Y. Sensitive immunosensor for cancer biomarker based on dual signal amplification strategy of graphene sheets and multienzyme functionalized carbon nanospheres. Anal. Chem 2010, 82, 2989–2995. [Google Scholar]
- Malhotra, R.; Patel, V.; Vaqué, J.P.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive electrochemical immunosensor for oral cancer biomarker IL-6 using carbon nanotube forest electrodes and multilabel amplification. Anal. Chem 2010, 82, 3118–3123. [Google Scholar]
- Cui, R.; Zhu, J.J. Fabrication of a novel electrochemical immunosensor based on the gold nanoparticles/colloidal carbon nanosphere hybrid material. Electrochim. Acta 2010, 55, 7814–7817. [Google Scholar]
- Xie, Y.; Yuan, R.; Chai, Y.Q. Immobilization of DNA-Hb on zirconia thin film modified gold electrode for preparation of a hydrogen peroxide biosensor. Chem. Sens 2008, 32–38. [Google Scholar]
- Tang, J.; Su, B.; Tang, D.; Chen, G. Conductive carbon nanoparticle-based electrochemical immunosensor with enhanced sensitivity for [alpha]-fetoprotein using irregular-shaped gold nanoparticles-labeled enzyme-linked antibodies as signal improvement. Biosens. Bioelectron 2010, 25, 2657–2662. [Google Scholar]
- Ou, C.; Yuan, R.; Chai, Y. A novel amperometric immunosensor based on layer-by-layer assembly of gold nanoparticles–multi-walled carbon nanotubes-thionine multilayer films on polyelectrolyte surface. Anal. Chim. Acta 2007, 603, 205–213. [Google Scholar]
- Hu, Y.; Carr, P.W. Synthesis and characterization of new zirconia-based polymeric cation-exchange stationary phases for high-performance liquid chromatography of proteins. Anal. Chem 1998, 70, 1934–1942. [Google Scholar]
- Sun, L.; McCormick, A.V.; Carr, P.W. Study of the irreversible adsorption of proteins on polybutadiene-coated zirconia. J. Chromatogr. A 1994, 658, 465–473. [Google Scholar]
- Wang, J.; Chen, Q.; Zeng, C.; Hou, B. Magnetic-field-induced growth of single-crystalline Fe3O4 nanowires. Adv. Mater 2004, 16, 137–140. [Google Scholar]
- Wu, Y.Z.; Xu, W.L.; Hou, J.G.; Gan, N.; Li, T.H. Determination of trace organophosphous pesticides in vegetables by inductively coupled plasma-atomic emission spectrometry based on magnetic enrichment by nano Fe3O4@ZrO2. Chin. J. Pestic. Sci 2010, 2, 178–184. [Google Scholar]
- Shang, L.B.; Liu, X.J.; Zhong, J.; Fan, C.H.; Suzuki, Iwao; Li, G.X. Fabrication of ultrathin, protein-containing films by layer-by-layer assembly electrochemical characterization of hemoglobin entrapped in the film. Chem. Lett 2003, 32, 296–297. [Google Scholar]
- Tang, D.P.; Yuan, R.; Chai, Y.Q. Ultrasensitive Electrochemical Immunosensor for Clinical Immunoassay Using Thionine-Doped Magnetic Gold Nanospheres as Labels and Horseradish Peroxidase as Enhancer. Anal Chem 2008, 80, 1582–1588. [Google Scholar]
- Zhang, X.T.; Wu, Y.F.; Tu, Y.F.; Liu, S.Q. A reusable electrochemical immunosensor for carcinoembryonic antigen via molecular recognition of glycoprotein antibody by phenylboronic acid self-assembly layer on gold. Analyst 2008, 133, 485–492. [Google Scholar]
- Lv, P.; Min, L.G.; Yuan, R.; Chai, Y.Q.; Chen, S.H. A novel immunosensor for carcinoembryonic antigen based on poly(diallyldimethylammonium chloride) protected prussian blue nanoparticles and double-layer nanometer-sized gold particles. Microchim. Acta 2010, 171, 297–304. [Google Scholar]
- Fu, X.H. Magnetic-controlled non-competitive enzyme-linked voltammetric immunoassay for carcinoembryonic antigen. Biochem. Eng. J 2008, 39, 267–275. [Google Scholar]
- Shi, W.T.; Ma, Z.F. A novel label-free amperometric immunosensor for carcinoembryonic antigen based on redox membrane. Biosens. Bioelectron 2011, 26, 3068–3071. [Google Scholar]
- Yuan, Y.R.; Yuan, R.; Chai, Y.Q.; Zhao, Y.; Miao, X.M. Electrochemical amperometric immunoassay for carcinoembryonic antigen based on bi-layer nano-Au and nickel hexacyanoferrates nanoparticles modified glassy carbon electrode. J. Electroanal. Chem 2009, 626, 6–13. [Google Scholar]
- Yuan, S.; Yuan, R.; Chai, Y.; Mao, L.; Yang, X.; Yuan, Y.; Niu, H. Sandwich-type electrochemiluminescence immunosensor based on Ru-silica@Au composite nanoparticles labeled anti-CEA. Talanta 2010, 82, 1468–1471. [Google Scholar]
- Wu, J.; Tang, J.; Dai, Z.; Yan, F.; Ju, H.; El Murr, N. A disposable electrochemical immunosensor for flow injection immunoassay of carcinoembryonic antigen. Biosens. Bioelectron 2006, 22, 102–108. [Google Scholar]
- Wu, Y.; Chen, C.; Liu, S. Enzyme-functionalized silica nanoparticles as sensitive labels in biosensing. Anal. Chem 2009, 81, 1600–1607. [Google Scholar]
- Wang, M.; Li, Z.; Gao, Y. Electrochemical analysis of pesticides by nano zirconia modified film electrode. Sens. Lett 2008, 6, 966–969. [Google Scholar]
- Huckel, M.; Wirth, H.J.; Hearn, M.T. Porous zirconia: A new support material for enzyme immobilization. J. Biochem. Biophys. Methods 1996, 31, 165–179. [Google Scholar]





| Immunosensor fabrication | Linear range (ng/mL) | DL (ng/mL) | ref. |
|---|---|---|---|
| HRP-anti-CEA/nano-Au/CS/CPCE | 0.5–25 | 0.22 | [19] |
| anti-CEA/protein A/nano Au/ carbon fiber microelectrode (CFME) | 0.01–160 | 0.005 | [20] |
| HRP–anti-CEA/nanogold/chitosan/SPCE | 2.5–40.0 | 1.1 | [21] |
| anti-CEA/double nano-Au/poly(diallyldimethylammonium chloride /GCE | 0.5–120 | 0.2 | [22] |
| HRP-anti-CEA/GA/CoFe2O4/CPE | 1.5–60 | 0.5 | [23] |
| anti-CEA/Au/Fe3O4 NPs/SPCE | 0.01–150 | 0.005 | [24] |
| anti-CEA/nano-Au/NiHCF/nano-Au/GCE | 0.5–160 | 0.1 | [25] |
| This method | 0.008–200 | 0.005 | Our work |
| CEA concentration (ng/mL) | |||||
|---|---|---|---|---|---|
| Sample | Detected by this method | Detected by ELISA | [CEA] Added in serum | Found after add CEA | Recovery (%) a |
| 1 | 2.1 | 2.0 | 2.0 | 4.3 | 105 |
| 2 | 4.2 | 4.0 | 4.0 | 8.0 | 95 |
| 3 | 10.2 | 10.5 | 10.0 | 21 | 107 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gan, N.; Jia, L.; Zheng, L. A Sandwich Electrochemical Immunosensor Using Magnetic DNA Nanoprobes for Carcinoembryonic Antigen. Int. J. Mol. Sci. 2011, 12, 7410-7423. https://doi.org/10.3390/ijms12117410
Gan N, Jia L, Zheng L. A Sandwich Electrochemical Immunosensor Using Magnetic DNA Nanoprobes for Carcinoembryonic Antigen. International Journal of Molecular Sciences. 2011; 12(11):7410-7423. https://doi.org/10.3390/ijms12117410
Chicago/Turabian StyleGan, Ning, Liyong Jia, and Lei Zheng. 2011. "A Sandwich Electrochemical Immunosensor Using Magnetic DNA Nanoprobes for Carcinoembryonic Antigen" International Journal of Molecular Sciences 12, no. 11: 7410-7423. https://doi.org/10.3390/ijms12117410




