The Impact of Membrane Lipid Composition on Macrophage Activation in the Immune Defense against Rhodococcus equi and Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results and Discussion
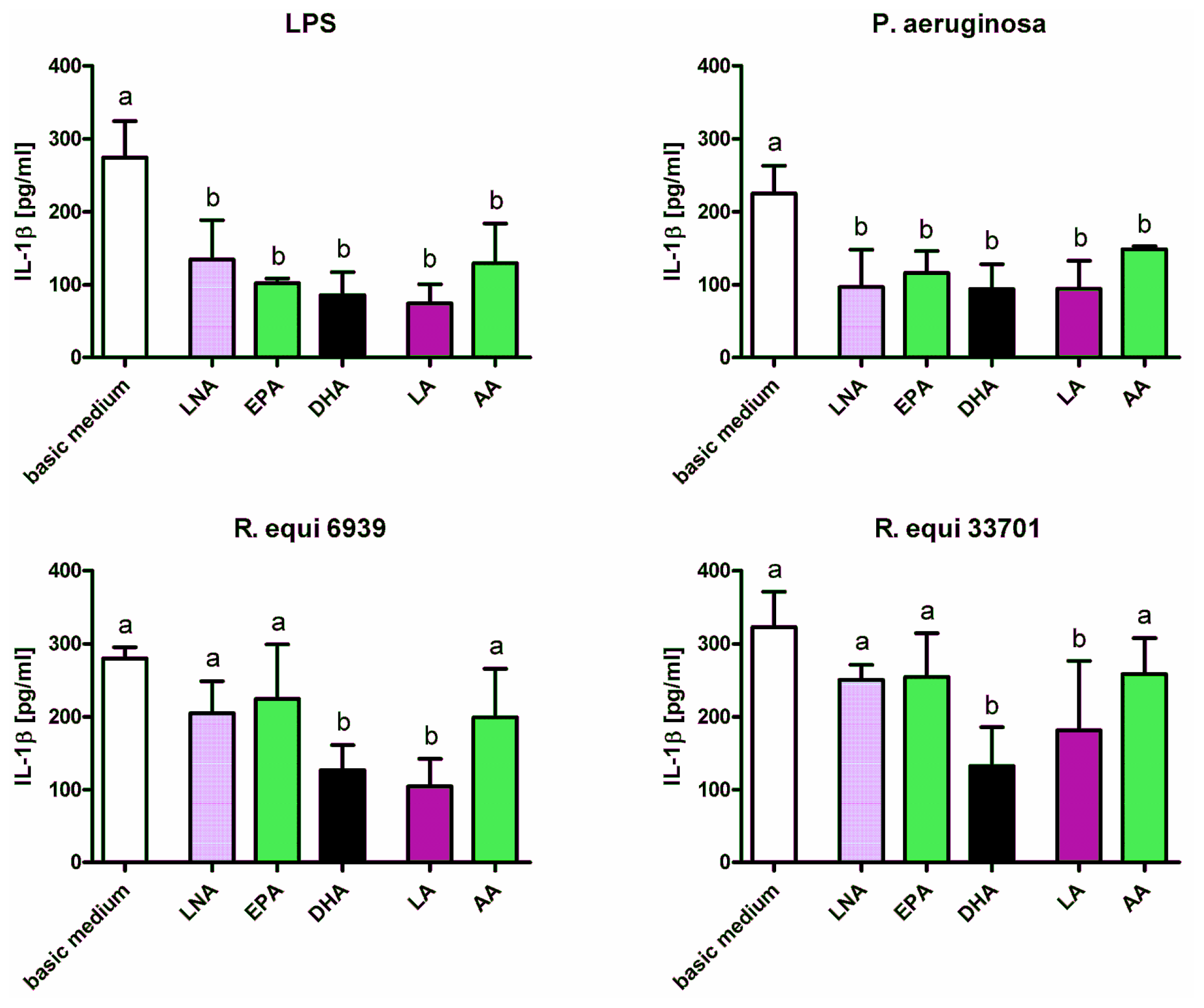
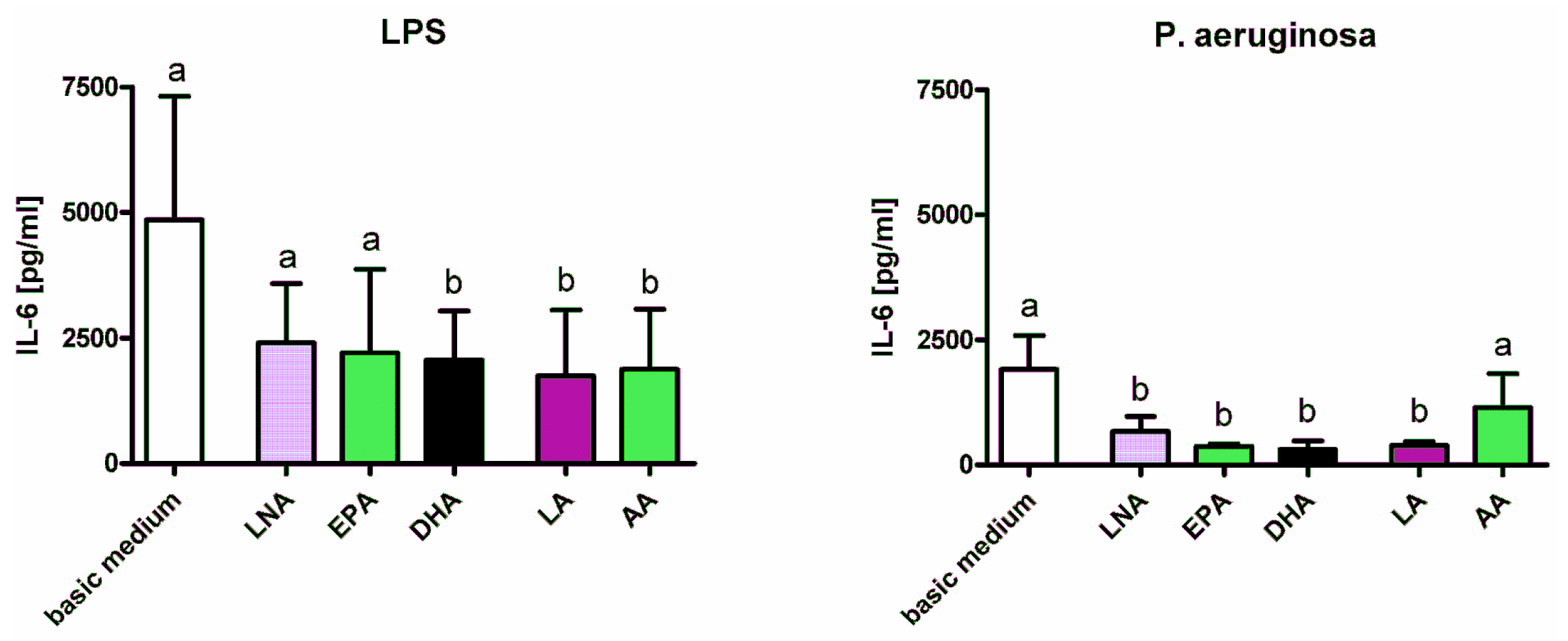
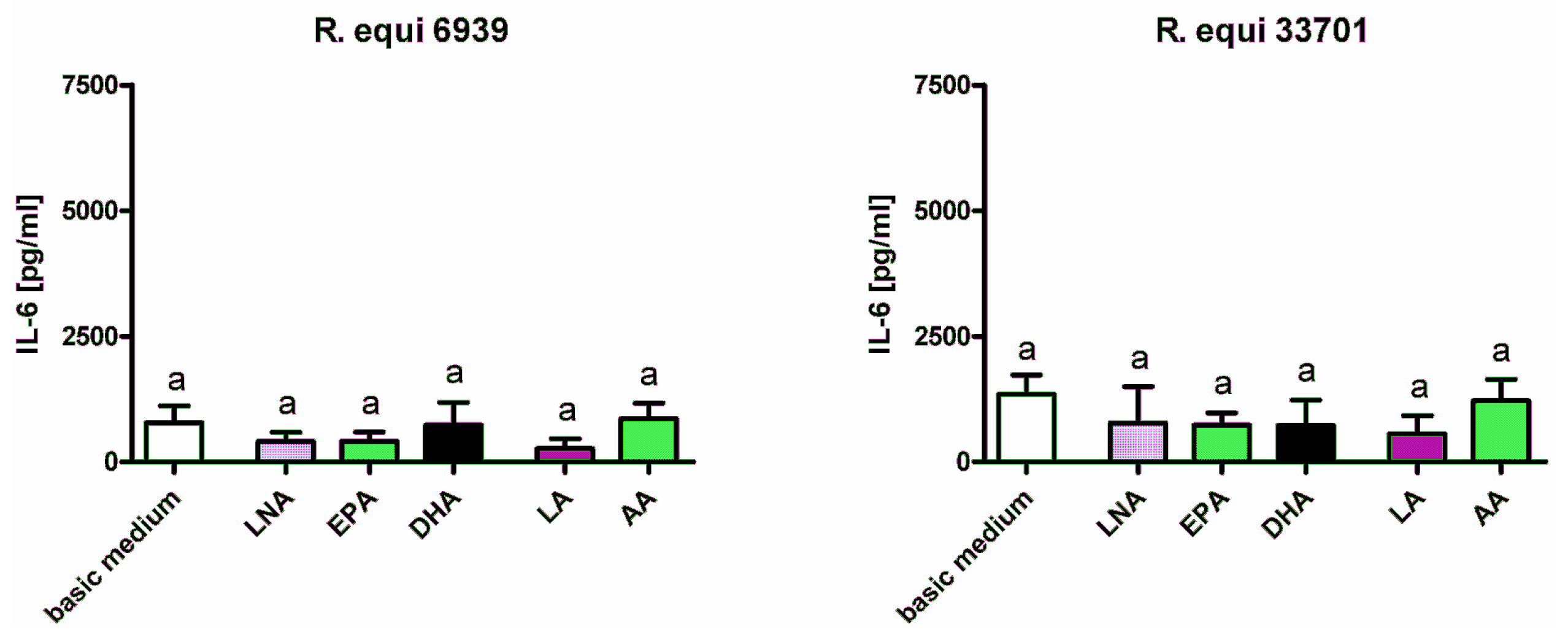
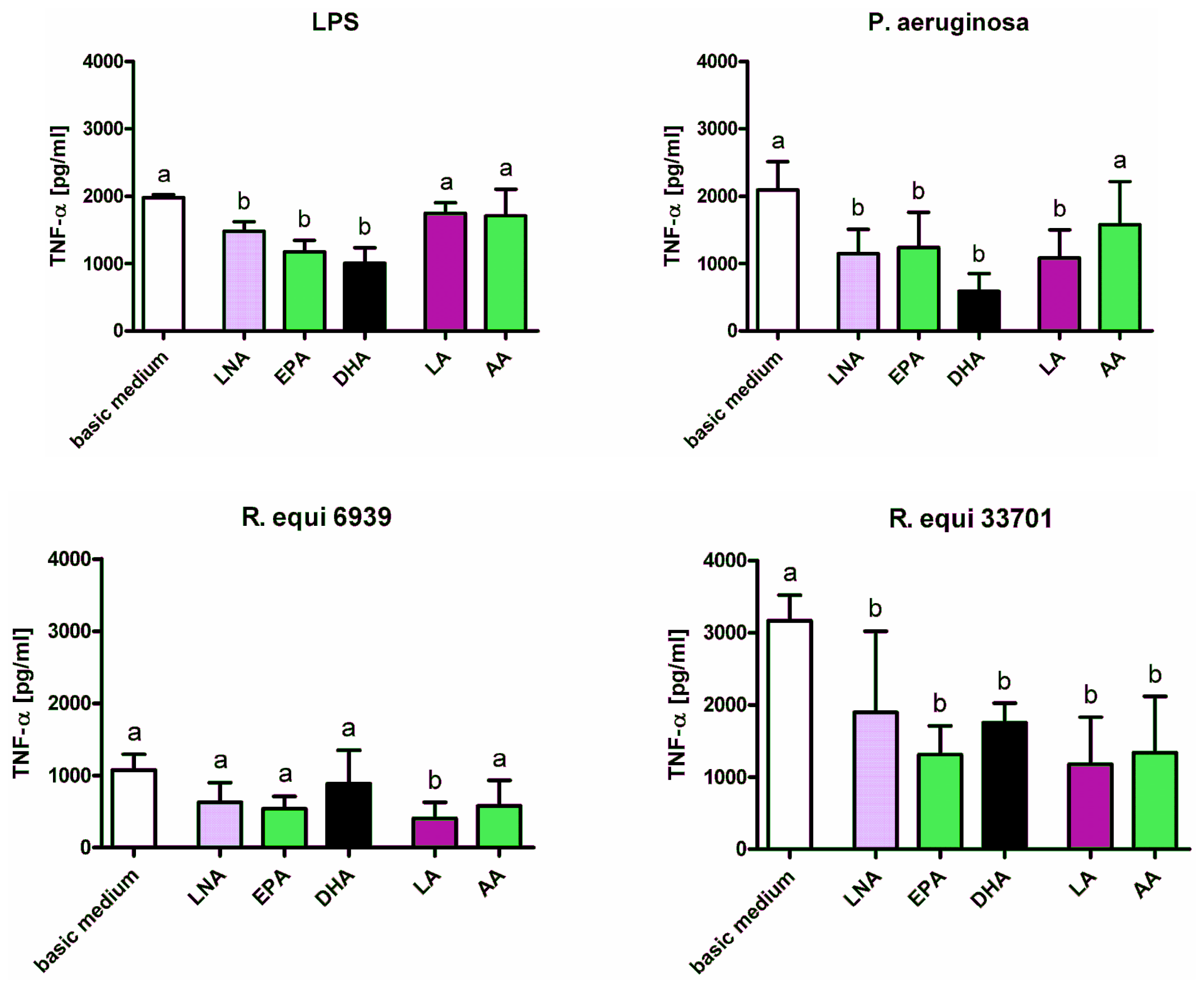
2.1. Cytokines
2.2. Surface Molecules
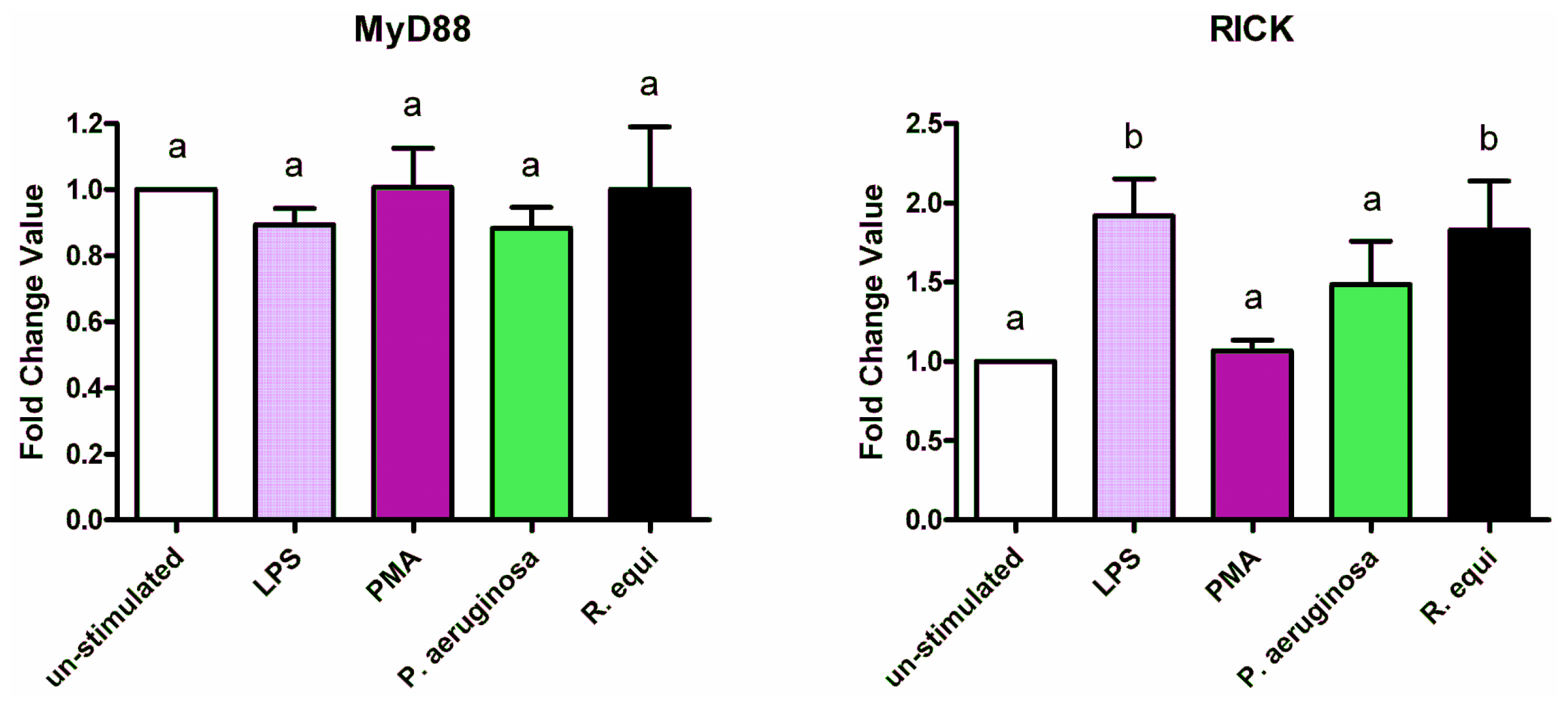
2.3. Adapter Proteins
2.4. Lysozyme
3. Experimental Section
3.1. Materials
3.2. Cell Culture
3.3. Cytokine Production
3.4. Gene Expression Analysis
3.5. Lysozyme Assay
3.6. CD86 Protein Expression
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar]
- Wassall, S.R.; Stillwell, W. Docosahexaenoic acid domains: The ultimate non-raft membrane domain. Chem. Phys. Lipids 2008, 153, 57–63. [Google Scholar]
- Chapkin, R.S.; Kim, W.; Lupton, J.R.; McMurray, D.N. Dietary docosahexaenoic and eicosapentaenoic acid: Emerging mediators of inflammation. Prostaglandins Leukot Essent. Fat. Acids 2009, 81, 187–191. [Google Scholar]
- Galli, C.; Calder, P.C. Effects of fat and fatty acid intake on inflammatory and immune responses: A critical review. Ann. Nutr. Metab 2009, 55, 123–139. [Google Scholar]
- Walloschke, B.; Fuhrmann, H.; Schumann, J. Enrichment of RAW264.7 macrophages with essential 18-carbon fatty acids affects both respiratory burst and production of immune modulating cytokines. J. Nutr. Biochem 2010, 21, 556–560. [Google Scholar]
- Schumann, J.; Fuhrmann, H. Impairment of NFkappaB activity by unsaturated fatty acids. Int. Immunopharmacol 2010, 10, 978–984. [Google Scholar]
- Schmutzler, S.; Bachmann, L.; Fuhrmann, H.; Schumann, J. PUFA-dependent alteration of oxidative parameters of a canine mastocytoma cell line. Acta Vet. Hung 2010, 58, 453–464. [Google Scholar]
- Groves, E.; Dart, A.E.; Covarelli, V.; Caron, E. Molecular mechanisms of phagocytic uptake in mammalian cells. Cell. Mol. Life Sci 2008, 65, 1957–1976. [Google Scholar]
- Bryant, C.; Fitzgerald, K.A. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol 2009, 19, 455–464. [Google Scholar]
- Russell, D.G.; Vanderven, B.C.; Glennie, S.; Mwandumba, H.; Heyderman, R.S. The macrophage marches on its phagosome: Dynamic assays of phagosome function. Nat. Rev. Immunol 2009, 9, 594–600. [Google Scholar]
- Hamilton, T.A.; Ohmori, Y.; Tebo, J.M.; Kishore, R. Regulation of macrophage gene expression by pro- and anti-inflammatory cytokines. Pathobiology 1999, 67, 241–244. [Google Scholar]
- Trautmann, M.; Lepper, P.M.; Haller, M. Ecology of Pseudomonas aeruginosa in the intensive care unit and the evolving role of water outlets as a reservoir of the organism. Am. J. Infect. Control 2005, 33, S41–S49. [Google Scholar]
- Prescott, J.F. Rhodococcus equi: An animal and human pathogen. Clin. Microbiol. Rev 1991, 4, 20–34. [Google Scholar]
- Kamboj, M.; Kalra, A.; Kak, V. Rhodococcus equi brain abscess in a patient without HIV. J. Clin. Pathol 2005, 58, 423–425. [Google Scholar]
- Bell, K.S.; Philp, J.C.; Aw, D.W.; Christofi, N. The genus Rhodococcus. J. Appl. Microbiol 1998, 85, 195–210. [Google Scholar]
- Fernandez-Mora, E.; Polidori, M.; Luhrmann, A.; Schaible, U.E.; Haas, A. Maturation of Rhodococcus equi-containing vacuoles is arrested after completion of the early endosome stage. Traffic 2005, 6, 635–653. [Google Scholar]
- von, B.K.; Haas, A. Molecular and infection biology of the horse pathogen Rhodococcus equi. FEMS Microbiol. Rev 2009, 33, 870–891. [Google Scholar]
- Blanc, D.S.; Petignat, C.; Janin, B.; Bille, J.; Francioli, P. Frequency and molecular diversity of Pseudomonas aeruginosa upon admission and during hospitalization: A prospective epidemiologic study. Clin. Microbiol. Infect 1998, 4, 242–247. [Google Scholar]
- Strateva, T.; Yordanov, D. Pseudomonas aeruginosa—A phenomenon of bacterial resistance. J. Med. Microbiol 2009, 58, 1133–1148. [Google Scholar]
- Pechere, J.C.; Kohler, T. Patterns and modes of beta-lactam resistance in Pseudomonas aeruginosa. Clin. Microbiol. Infect 1999, 5, S15–S18. [Google Scholar]
- Boyen, F.; Eeckhaut, V.; Van, I.F.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F. Quorum sensing in veterinary pathogens: Mechanisms, clinical importance and future perspectives. Vet. Microbiol 2009, 135, 187–195. [Google Scholar]
- Veesenmeyer, J.L.; Hauser, A.R.; Lisboa, T.; Rello, J. Pseudomonas aeruginosa virulence and therapy: Evolving translational strategies. Crit. Care Med 2009, 37, 1777–1786. [Google Scholar]
- Darrah, P.A.; Monaco, M.C.; Jain, S.; Hondalus, M.K.; Golenbock, D.T.; Mosser, D.M. Innate immune responses to Rhodococcus equi. J. Immunol 2004, 173, 1914–1924. [Google Scholar]
- Weldon, S.M.; Mullen, A.C.; Loscher, C.E.; Hurley, L.A.; Roche, H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem 2007, 18, 250–258. [Google Scholar]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem 2010, 21, 444–450. [Google Scholar]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar]
- Mitchell, J.A.; Paul-Clark, M.J.; Clarke, G.W.; McMaster, S.K.; Cartwright, N. Critical role of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. J. Endocrinol 2007, 193, 323–330. [Google Scholar]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol 2011, 30, 16–34. [Google Scholar]
- Park, J.H.; Kim, Y.G.; Nunez, G. RICK promotes inflammation and lethality after Gram-negative bacterial infection in mice stimulated with lipopolysaccharide. Infect. Immun 2009, 77, 1569–1578. [Google Scholar]
- Olsson, S.; Sundler, R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol. Immunol 2006, 43, 607–612. [Google Scholar]
- Wong, S.W.; Kwon, M.J.; Choi, A.M.; Kim, H.P.; Nakahira, K.; Hwang, D.H. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem 2009, 284, 27384–27392. [Google Scholar]
- Bishop-Bailey, D.; Bystrom, J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol. Ther 2009, 124, 141–150. [Google Scholar]
- Lee, J.Y.; Ye, J.; Gao, Z.; Youn, H.S.; Lee, W.H.; Zhao, L.; Sizemore, N.; Hwang, D.H. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J. Biol. Chem 2003, 278, 37041–37051. [Google Scholar]
- Martinez, J.G.; Waldon, M.; Huang, Q.; Alvarez, S.; Oren, A.; Sandoval, N.; Du, M.; Zhou, F.; Zenz, A.; Lohner, K.; et al. Membrane-targeted synergistic activity of docosahexaenoic acid and lysozyme against Pseudomonas aeruginosa. Biochem. J 2009, 419, 193–200. [Google Scholar]
- Helal, R.; Melzig, M.F. New aspects in the synthesis and secretion of lysozyme by cultured human monocyte cell lines. In Vitro. Cell. Dev. Biol. Anim 2010, 46, 492–496. [Google Scholar]
- Leaf, D.A.; Connor, W.E.; Barstad, L.; Sexton, G. Incorporation of dietary n-3 fatty acids into the fatty acids of human adipose tissue and plasma lipid classes. Am. J. Clin. Nutr 1995, 62, 68–73. [Google Scholar]









© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schoeniger, A.; Adolph, S.; Fuhrmann, H.; Schumann, J. The Impact of Membrane Lipid Composition on Macrophage Activation in the Immune Defense against Rhodococcus equi and Pseudomonas aeruginosa. Int. J. Mol. Sci. 2011, 12, 7510-7528. https://doi.org/10.3390/ijms12117510
Schoeniger A, Adolph S, Fuhrmann H, Schumann J. The Impact of Membrane Lipid Composition on Macrophage Activation in the Immune Defense against Rhodococcus equi and Pseudomonas aeruginosa. International Journal of Molecular Sciences. 2011; 12(11):7510-7528. https://doi.org/10.3390/ijms12117510
Chicago/Turabian StyleSchoeniger, Axel, Stephanie Adolph, Herbert Fuhrmann, and Julia Schumann. 2011. "The Impact of Membrane Lipid Composition on Macrophage Activation in the Immune Defense against Rhodococcus equi and Pseudomonas aeruginosa" International Journal of Molecular Sciences 12, no. 11: 7510-7528. https://doi.org/10.3390/ijms12117510



