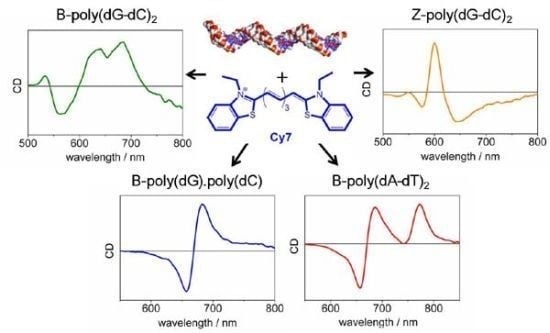
3,3′-Diethylthiatricarbocyanine Iodide: A Highly Sensitive Chiroptical Reporter of DNA Helicity and Sequence
Abstract
:1. Introduction
2. Results and Discussion
2.1. UV-vis Spectroscopy of the B-forms of poly(dA-dT)2, poly(dC).poly(dG), and poly(dG-dC)2
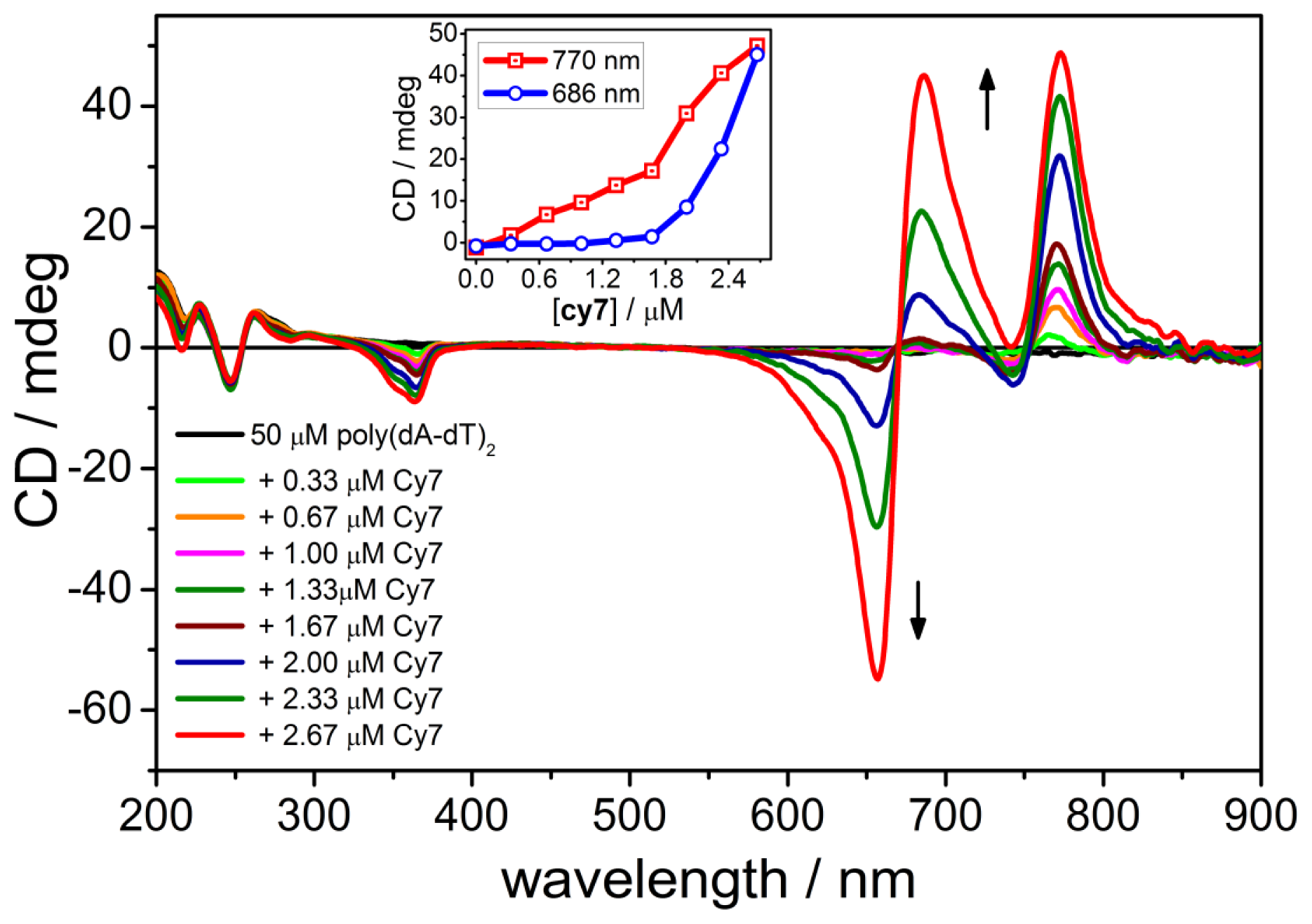
2.2. CD Spectroscopy of the B-form of poly(dA-dT)2
2.3. CD Spectroscopy of the B-form of poly(dC).poly(dG)
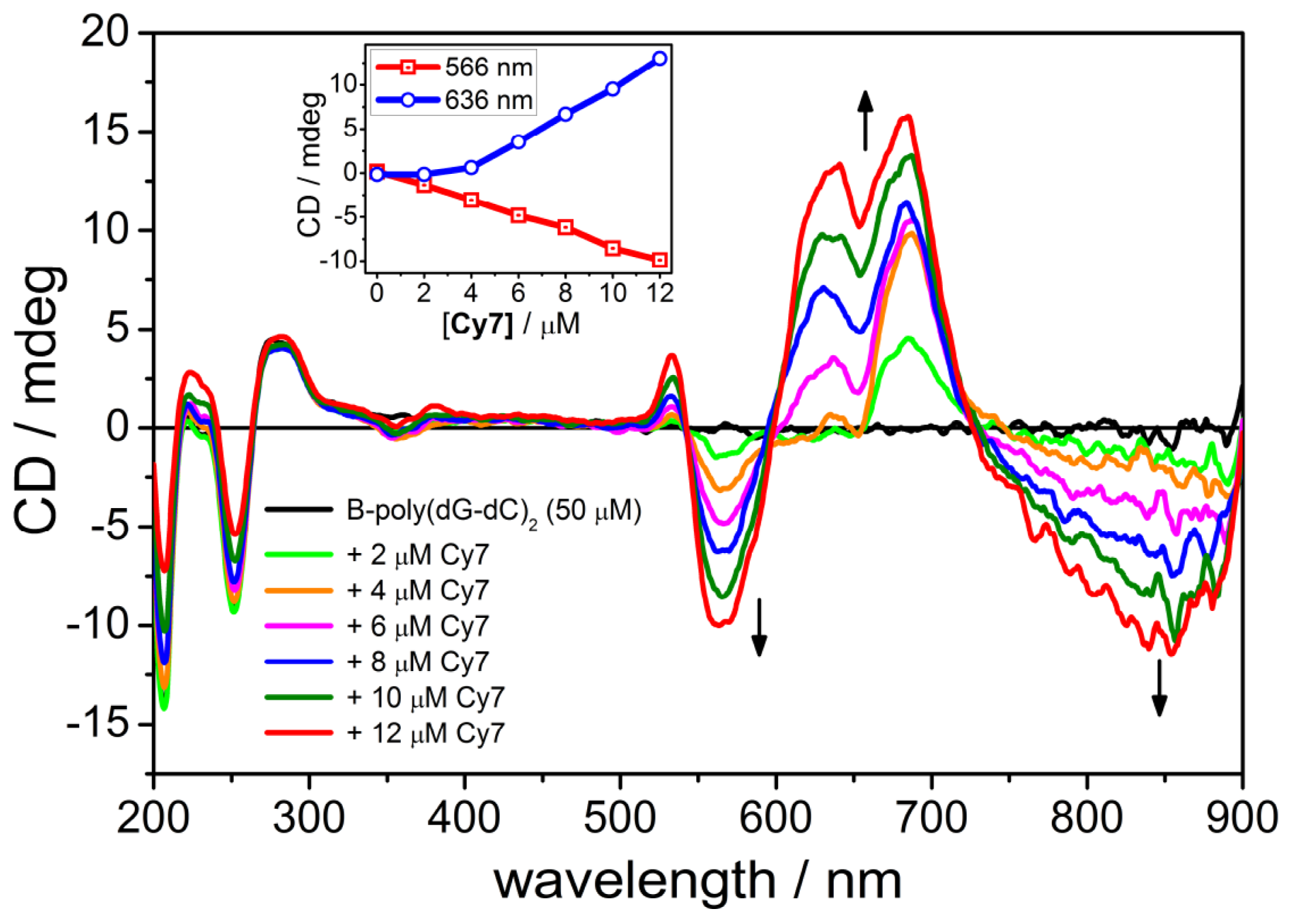
2.4. CD Spectroscopy of the B-form of poly(dC-dG)2
2.5. UV-vis and CD Spectroscopies of the Z-form of poly(dC-G)2
3. Experimental Section
4. Conclusions
Supplementary Material
ijms-12-08052-s001.pdfAcknowledgments
References
- Brooker, L.G.S.; Sprague, R.H.; Smyth, C.P.; Lewis, G.L. Color and constitution I Halochromism of anhydronium bases related to the cyanine dyes. J. Am. Chem. Soc 1940, 62, 1116–1125. [Google Scholar]
- Byers, G.W.; Gross, S.; Henrichs, P.M. Direct and sensitized photooxidation of cyanine dyes. Photochem. Photobiol 1976, 23, 37–43. [Google Scholar]
- Yang, S.J.; Tian, H.; Xiao, H.M.; Shang, X.H.; Gong, X.D.; Yao, S.D.; Chen, K.C. Photodegradation of cyanine and merocyanine dyes. Dyes Pigments 2001, 49, 93–101. [Google Scholar]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A review. Chem. Rev 2000, 100, 1973–2011. [Google Scholar]
- Mustroph, H.; Stollenwerk, M.; Bressau, V. Current developments in optical data storage with organic dyes. Angew. Chem. Int. Ed 2006, 45, 2016–2035. [Google Scholar]
- Gomez-Hens, A.; Aguilar-Caballos, M.P. Long-wavelength fluorophores: New trends in their analytical use. TrAC Trends Anal. Chem 2004, 23, 127–136. [Google Scholar]
- Webster, S.; Fu, J.; Padilha, L.A.; Przhonska, O.V.; Hagan, D.J.; Van Stryland, E.W.; Bondar, M.V.; Slominsky, Y.L.; Kachkovski, A.D. Comparison of nonlinear absorption in three similar dyes: Polymethine, squaraine and tetraone. Chem. Phys 2008, 348, 143–151. [Google Scholar]
- Flanagan, J.H.; Khan, S.H.; Menchen, S.; Soper, S.A.; Hammer, R.P. Functionalized tricarbocyanine dyes as near-infrared fluorescent probes for biomolecules. Bioconjugate Chem 1997, 8, 751–756. [Google Scholar]
- Garoff, R.A.; Litzinger, E.A.; Connor, R.E.; Fishman, I.; Armitage, B.A. Helical aggregation of cyanine dyes on DNA templates: Effect of dye structure on formation of homo- and heteroaggregates. Langmuir 2002, 18, 6330–6337. [Google Scholar]
- Hannah, K.C.; Armitage, B.A. DNA-templated assembly of helical cyanine dye aggregates: A supramolecular chain polymerization. Acc. Chem. Res 2004, 37, 845–853. [Google Scholar]
- Wang, M.M.; Silva, G.L.; Armitage, B.A. DNA-templated formation of a helical cyanine dye J-aggregate. J. Am. Chem. Soc 2000, 122, 9977–9986. [Google Scholar]
- Hannah, K.C.; Gil, R.R.; Armitage, B.A. H-1 NMR and optical spectroscopic investigation of the sequence-dependent dimerization of a symmetrical cyanine dye in the DNA minor groove. Biochemistry 2005, 44, 15924–15929. [Google Scholar]
- Seifert, J.L.; Connor, R.E.; Kushon, S.A.; Wang, M.; Armitage, B.A. Spontaneous assembly of helical cyanine dye aggregates on DNA nanotemplates. J. Am. Chem. Soc 1999, 121, 2987–2995. [Google Scholar]
- Barton, J.K.; Basile, L.A.; Danishefsky, A.; Alexandrescu, A. Chiral probes for the handedness of DNA helices-enantiomers of tris(4,7-diphenylphenanthroline)ruthenium(Ii). Proc. Natl. Acad. Sci. USA 1984, 81, 1961–1965. [Google Scholar]
- Xu, Y.; Zhang, Y.X.; Sugiyama, H.; Umano, T.; Osuga, H.; Tanaka, K. (P)-Helicenes displays chiral selection in binding to Z-DNA. J. Am. Chem. Soc. 2004, 126, 6566–6567. [Google Scholar]
- Balaz, M.; de Napoli, M.; Holmes, A.E.; Mammana, A.; Nakanishi, K.; Berova, N.; Purrello, R. A Cationic zinc porphyrin as a chiroptical sensor for Z-DNA. Angew. Chem. Int. Ed 2005, 44, 4006–4009. [Google Scholar]
- Seo, Y.J.; Kim, B.H. Probing the B-to-Z-DNA duplex transition using terminally stacking ethynyl pyrene-modified adenosine and uridine bases. Chem. Commun 2006, 150–152. [Google Scholar]
- Balaz, M.; Li, B.C.; Steinkguger, J.D.; Ellestad, G.A.; Nakanishi, K.; Berova, N. Porphyrins conjugated to DNA as CD reporters of the salt-induced B to Z-DNA transition. Org. Biomol. Chem 2006, 4, 1865–1867. [Google Scholar]
- Kim, Y.-G.; Lowenhaupt, K.; Maas, S.; Herbert, A.; Schwartz, T.; Rich, A. The Zab domain of the human RNA editing enzyme ADAR1 recognizes Z-DNA when surrounded by B-DNA. J. Biol. Chem 2000, 275, 26828–26833. [Google Scholar]
- Herbert, A.; Rich, A. The role of binding domains for dsRNA and Z-DNA in the in vivo editing of minimal substrates by ADAR1. Proc. Natl. Acad. Sci. USA 2001, 98, 12132–12137. [Google Scholar]
- Kim, Y.-G.; Lowenhaupt, K.; Oh, D.-B.; Kim, K.K.; Rich, A. Evidence that vaccinia virulence faktor E3L binds to Z-DNA in vivo-Implications for development of a therapy for poxvirus infection. Proc. Natl. Acad. Sci. USA 2004, 101, 1514–1518. [Google Scholar]
- Champ, P.C.; Maurice, S.; Vargason, J.M.; Camp, T.; Ho, P.S. Distributions of Z-DNA and nuclear factor I in human chromosome 22: A model for coupled transcriptional regulation. Nucleic Acids Res 2004, 32, 6501–6510. [Google Scholar]
- Rich, A.; Zhang, S. Z-DNA: The long road to biological function. Nat. Rev. Genet 2003, 4, 566–572. [Google Scholar]
- Herbert, A.; Rich, A. Left-handed Z-DNA: Structure and function. Genetica 1999, 106, 37–47. [Google Scholar]
- D’Urso, A.; Mammana, A.; Balaz, M.; Holmes, A.E.; Berova, N.; Lauceri, R.; Purrello, R. Interactions of a tetraanionic porphyrin with DNA: From a Z-DNA sensor to a versatile supramolecular device. J. Am. Chem. Soc 2009, 131, 2046–2047. [Google Scholar]
- D’Urso, A.; Kyu Choi, J.; Shabbir-Hussain, M.; Ngwa, F.N.; Lambousis, M.I.; Purrello, R.; Balaz, M. Recognition of left-handed Z-DNA of short unmodified oligonucleotides under physiological ionic strength conditions. Biochem. Biophys. Res. Commun 2010, 397, 329–332. [Google Scholar]
- Choi, J.K.; Sargsyan, G.; Shabbir-Hussain, M.; Holmes, A.E.; Balaz, M. Chiroptical detection of condensed Nickel(II)-Z-DNA in the presence of the B-DNA via porphyrin exciton coupled circular dichroism. J. Phys. Chem. B 2011, 115, 10182–10188. [Google Scholar]
- D’Urso, A.; Holmes, A.E.; Berova, N.; Balaz, M.; Purrello, R. Z-DNA recognition in B-Z-B sequences by a cationic zinc porphyrin. Chem. Asian J 2011, 6, 3104–3109. [Google Scholar]
- Chow, C.S.; Barton, J.K. Transition metal complexes as probes of nucleic acids. Methods Enzymol 1992, 212, 219–242. [Google Scholar]
- Barton, J.K.; Lolis, E. Chiral discrimination in the covalent binding of bis(phenanthroline)dichlororuthenium(II) to B-DNA. J. Am. Chem. Soc 1985, 107, 708–709. [Google Scholar]
- Barton, J.K.; Dannenberg, J.J.; Raphael, A.L. Enantiomeric selectivity in binding tris(phenanthroline)zinc(II) to DNA. J. Am. Chem. Soc 1982, 104, 4967–4969. [Google Scholar]
- Parkinson, A.; Hawken, M.; Hall, M.; Sanders, K.J.; Rodger, A. Amine induced Z-DNA in poly(dG-dC). poly(dG-dC): Circular dichroism and gel electrophoresis study. Phys. Chem. Chem. Phys 2000, 2, 5469–5478. [Google Scholar]
- Sheppard, S.E.; Geddes, A.L. Effect of solvents upon the absorption spectra of dyes. IV. Water as solvent: A common pattern. J. Am. Chem. Soc 1944, 66, 1995–2002. [Google Scholar]






© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Choi, J.K.; D’Urso, A.; Trauernicht, M.; Shabbir-Hussain, M.; Holmes, A.E.; Balaz, M. 3,3′-Diethylthiatricarbocyanine Iodide: A Highly Sensitive Chiroptical Reporter of DNA Helicity and Sequence. Int. J. Mol. Sci. 2011, 12, 8052-8062. https://doi.org/10.3390/ijms12118052
Choi JK, D’Urso A, Trauernicht M, Shabbir-Hussain M, Holmes AE, Balaz M. 3,3′-Diethylthiatricarbocyanine Iodide: A Highly Sensitive Chiroptical Reporter of DNA Helicity and Sequence. International Journal of Molecular Sciences. 2011; 12(11):8052-8062. https://doi.org/10.3390/ijms12118052
Chicago/Turabian StyleChoi, Jung Kyu, Alessandro D’Urso, Mitch Trauernicht, Murtaza Shabbir-Hussain, Andrea E. Holmes, and Milan Balaz. 2011. "3,3′-Diethylthiatricarbocyanine Iodide: A Highly Sensitive Chiroptical Reporter of DNA Helicity and Sequence" International Journal of Molecular Sciences 12, no. 11: 8052-8062. https://doi.org/10.3390/ijms12118052