Protective Effect of the Ethyl Acetate Fraction of Sargassum muticum against Ultraviolet B–Irradiated Damage in Human Keratinocytes
Abstract
:1. Introduction
2. Results
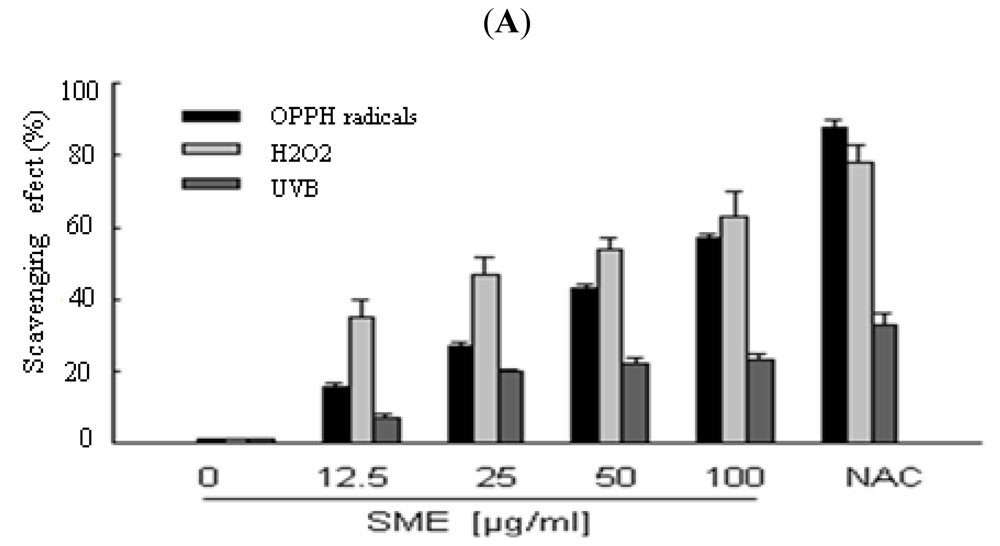
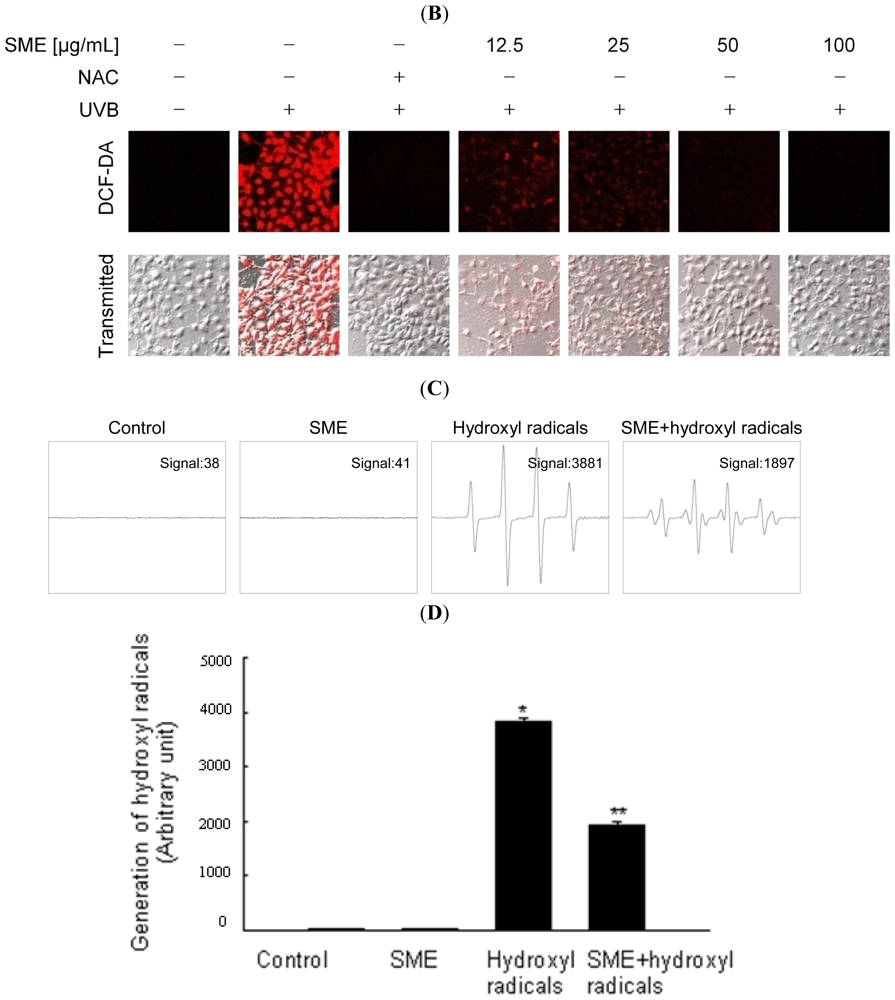
2.1. Scavenging Effect of SME toward Free Radicals
2.2. Effect of SME on Antioxidant Enzymes
2.3. Effect of SME against Cell Death Induced by UVB Radiation
3. Discussion
4. Experimental Section
4.1. Solvent Extraction of Sargassum Muticum
4.2. Reagents
4.3. Cell Culture
4.4. DPPH Radical Scavenging Activity
4.5. Intracellular ROS Scavenging Activity
4.6. Detection of Hydroxyl Radicals
4.7. Lipid Peroxidation Assay
4.8. Western Blot Analysis
4.9. SOD Activity
4.10. CAT Activity
4.11. Cell Viability Assay
4.12. Nuclear Staining with Hoechst 33342
4.13. Terminal Deoxynucleotidyl Transferase-Mediated Digoxigenin-dUTP Nick end Labeling (TUNEL) Assay
4.14. DNA Fragmentation
4.15. Statistical Analysis
5. Conclusion
Acknowledgements
References
- Chalam, K.V.; Khetpal, V.; Rusovici, R.; Balaiya, S. Role of ultraviolet radiation in age-related macular degeneration. Eye Contact Lens 2011, 37, 225–232. [Google Scholar]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol 2010, 49, 978–986. [Google Scholar]
- Halliday, G.M. Common links among the pathways leading to UV-induced immunosuppression. J. Invest. Dermatol 2010, 130, 1209–1212. [Google Scholar]
- Sime, S.; Reeve, V.E. Protection from inflammation, immunosuppression and carcinogenesis induced by UV radiation in mice by topical pycnogenol. Photochem. Photobiol 2004, 79, 193–198. [Google Scholar]
- Halliday, G.M.; Russo, P.A.; Yuen, K.S.; Robertson, B.O. Effect of inhibitors of oxygen radical and nitric oxide formation on UV radiation-induced erythema, immunosuppression and carcinogenesis. Redox Rep 1999, 4, 316–318. [Google Scholar]
- Chen, W.; Kang, J.; Xia, J.; Li, Y.; Yang, B.; Chen, B.; Sun, W.; Song, X.; Xiang, W.; Wang, X.; et al. p53-related apoptosis resistance and tumor suppression activity in UVB-induced premature senescent human skin fibroblasts. Int. J. Mol. Med 2008, 21, 645–653. [Google Scholar]
- Karol, M.H. Invited review: How environmental agents influence the aging process. Biomol. Ther 2009, 17, 113–124. [Google Scholar]
- Yasui, H.; Hakozaki, T.; Date, A.; Yoshii, T.; Sakurai, H. Real-time chemiluminescent imaging and detection of reactive oxygen species generated in the UVB-exposed human skin equivalent model. Biochem. Biophys. Res. Commun 2006, 347, 83–88. [Google Scholar]
- Masaki, H.; Sakurai, H. Increased generation of hydrogen peroxide possibly from mitochondrial respiratory chain after UVB irradiation of murine fibroblasts. J. Dermatol. Sci 1997, 14, 207–216. [Google Scholar]
- Ramachandran, S.; Rajendra Prasad, N.; Karthikeyan, S. Sesamol inhibits UVB-induced ROS generation and subsequent oxidative damage in cultured human skin dermal fibroblasts. Arch. Dermatol. Res 2010, 302, 733–744. [Google Scholar]
- Takahashi, H.; Hashimoto, Y.; Aoki, N.; Kinouchi, M.; Ishida-Yamamoto, A.; Iizuka, H. Copper, zinc-superoxide dismutase protects from ultraviolet B-induced apoptosis of SV40-transformed human keratinocytes: The protection is associated with the increased levels of antioxidant enzymes. J. Dermatol. Sci 2000, 23, 12–21. [Google Scholar]
- Decraene, D.; Smaers, K.; Gan, D.; Mammone, T.; Matsui, M.; Maes, D.; Declercq, L.; Garmyn, M. A synthetic superoxide dismutase/catalase mimetic (EUK-134) inhibits membrane-damage-induced activation of mitogen-activated protein kinase pathways and reduces p53 accumulation in ultraviolet B-exposed primary human keratinocytes. J. Invest. Dermatol 2004, 122, 484–491. [Google Scholar]
- Waster, P.K.; Ollinger, K.M. Redox-dependent translocation of p53 to mitochondria or nucleus in human melanocytes after UVA- and UVB-induced apoptosis. J. Invest. Dermatol 2009, 129, 1769–1781. [Google Scholar]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.H.; Khan, N.; Zaid, M.A.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Invest. Dermatol 2007, 127, 222–232. [Google Scholar]
- Ahn, S.M.; Hwang, J.S.; Lee, S.H. Fructose 1,6-diphosphate alleviates UV-induced oxidative skin damage in hairless mice. Biol. Pharm. Bull 2007, 30, 692–697. [Google Scholar]
- Mantena, S.K.; Katiyar, S.K. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-kappaB signaling in human epidermal keratinocytes. Free Radic. Biol. Med 2006, 40, 1603–1614. [Google Scholar]
- Kim, J.Y.; Lee, J.A.; Kim, K.N.; Yoon, W.J.; Lee, W.J.; Park, S.Y. Antioxidative and antimicrobial activities of sargassum muticum extracts. J. Korean Soc. Food Sci. Nutr 2007, 36, 663–669. [Google Scholar]
- Yoon, W.J.; Ham, Y.M.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Brown alga sargassum muticum inhibits proinflammatory cytokines, iNOS, and COX-2 expression in macrophage RAW 264.7 cells. Turk. J. Biol 2010, 34, 25–34. [Google Scholar]
- Okimotoa, Y.; Watanabea, A.; Nikia, E.; Yamashitab, T.; Noguchia, N. A novel fluorescent probe diphenyl-1-pyrenylphosphine to follow lipid peroxidation in cell membranes. FEBS Lett 2000, 474, 137–140. [Google Scholar]
- Scharffetter-Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol 2000, 35, 307–316. [Google Scholar]
- García-Casal, M.N.; Ramírez, J.; Leets, I.; Pereira, A.C.; Quiroga, M.F. Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp. and Porphyra sp.) in human subjects. Br. J. Nutr 2009, 101, 79–85. [Google Scholar]
- Shibata, T.; Yamaguchi, K.; Nagamura, K.; Kawaguchi, S.; Nagamura, T. Inhibitory activity of brown algae phlorotannins against glycosidases from the viscera of the turban shell Turbo cornutus. Eur. J. Phycol 2002, 37, 493–500. [Google Scholar]
- Kim, J.A.; Lee, J.M.; Shin, D.B.; Lee, N.H. The antioxidant activity and tyrosinase inhibitory activity of phlorotannins in Ecklonia cava. Food Sci. Biotechnol 2004, 13, 476–480. [Google Scholar]
- Nakamura, T.; Nagayama, K.; Uchida, K.; Tanaka, R. Antioxidant activity of phlorotannins isolated from the brown alga Eisenia bicyclis. Fish. Sci 1996, 62, 923–926. [Google Scholar]
- Kang, K.A.; Lee, K.H.; Chae, S.; Koh, Y.S.; Yoo, B.S.; Kim, J.H.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic. Res 2005, 39, 883–892. [Google Scholar]
- Filip, A.; Daicoviciu, D.; Clichici, S.; Mocan, T.; Muresan, A.; Postescu, I.D. Photoprotective effects of two natural products on ultraviolet B-induced oxidative stress and apoptosis in SKH-1 mouse skin. J. Med. Food 2011, 14, 761–766. [Google Scholar]
- Bozzo, C.; Tiberio, R.; Graziola, F.; Pertusi, G.; Valente, G.; Colombo, E.; Small, P.L.C.; Leigheb, G. A Mycobacterium ulcerans toxin, mycolactone, induces apoptosis in primary human keratinocytes and in HaCaT cells. Microb. Infect 2010, 12, 1258–1263. [Google Scholar]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar]
- Li, L.; Abe, Y.; Mashino, T.; Mochizuki, M.; Miyata, N. Signal enhancement in ESR spin-trapping for hydroxyl radicals. Anal. Sci 2003, 19, 1083–1084. [Google Scholar]
- Li, L.; Abe, Y.; Kanagawa, K.; Usui, N.; Imai, K.; Mashino, T.; Mochizuki, M.; Miyata, N. Distinguishing the 5, 5-dimethyl-1-pyrroline N-oxide (DMPO)-OH radical quenching effect from the hydroxyl radical scavenging effect in the ESR spin-trapping method. Anal. Chim. Acta 2004, 512, 121–124. [Google Scholar]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res 1987, 47, 936–942. [Google Scholar]
- Zheng, J.N.; Sun, Y.F.; Pei, D.S.; Liu, J.J.; Chen, J.C.; Li, W.; Sun, X.Q.; Shi, Q.D.; Han, R.F.; Ma, T.X. Inhibition of proliferation and induction of apoptosis in human renal carcinoma cells by anti-telomerase small interfering RNAs. Acta Biochim. Biophys. Sin. (Shanghai) 2006, 38, 500–506. [Google Scholar]





© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Piao, M.J.; Yoon, W.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Kim, D.S.; Lee, N.H.; Hyun, J.W. Protective Effect of the Ethyl Acetate Fraction of Sargassum muticum against Ultraviolet B–Irradiated Damage in Human Keratinocytes. Int. J. Mol. Sci. 2011, 12, 8146-8160. https://doi.org/10.3390/ijms12118146
Piao MJ, Yoon WJ, Kang HK, Yoo ES, Koh YS, Kim DS, Lee NH, Hyun JW. Protective Effect of the Ethyl Acetate Fraction of Sargassum muticum against Ultraviolet B–Irradiated Damage in Human Keratinocytes. International Journal of Molecular Sciences. 2011; 12(11):8146-8160. https://doi.org/10.3390/ijms12118146
Chicago/Turabian StylePiao, Mei Jing, Weon Jong Yoon, Hee Kyoung Kang, Eun Sook Yoo, Young Sang Koh, Dong Sam Kim, Nam Ho Lee, and Jin Won Hyun. 2011. "Protective Effect of the Ethyl Acetate Fraction of Sargassum muticum against Ultraviolet B–Irradiated Damage in Human Keratinocytes" International Journal of Molecular Sciences 12, no. 11: 8146-8160. https://doi.org/10.3390/ijms12118146



