Active Protein Aggregates Produced in Escherichia coli
Abstract
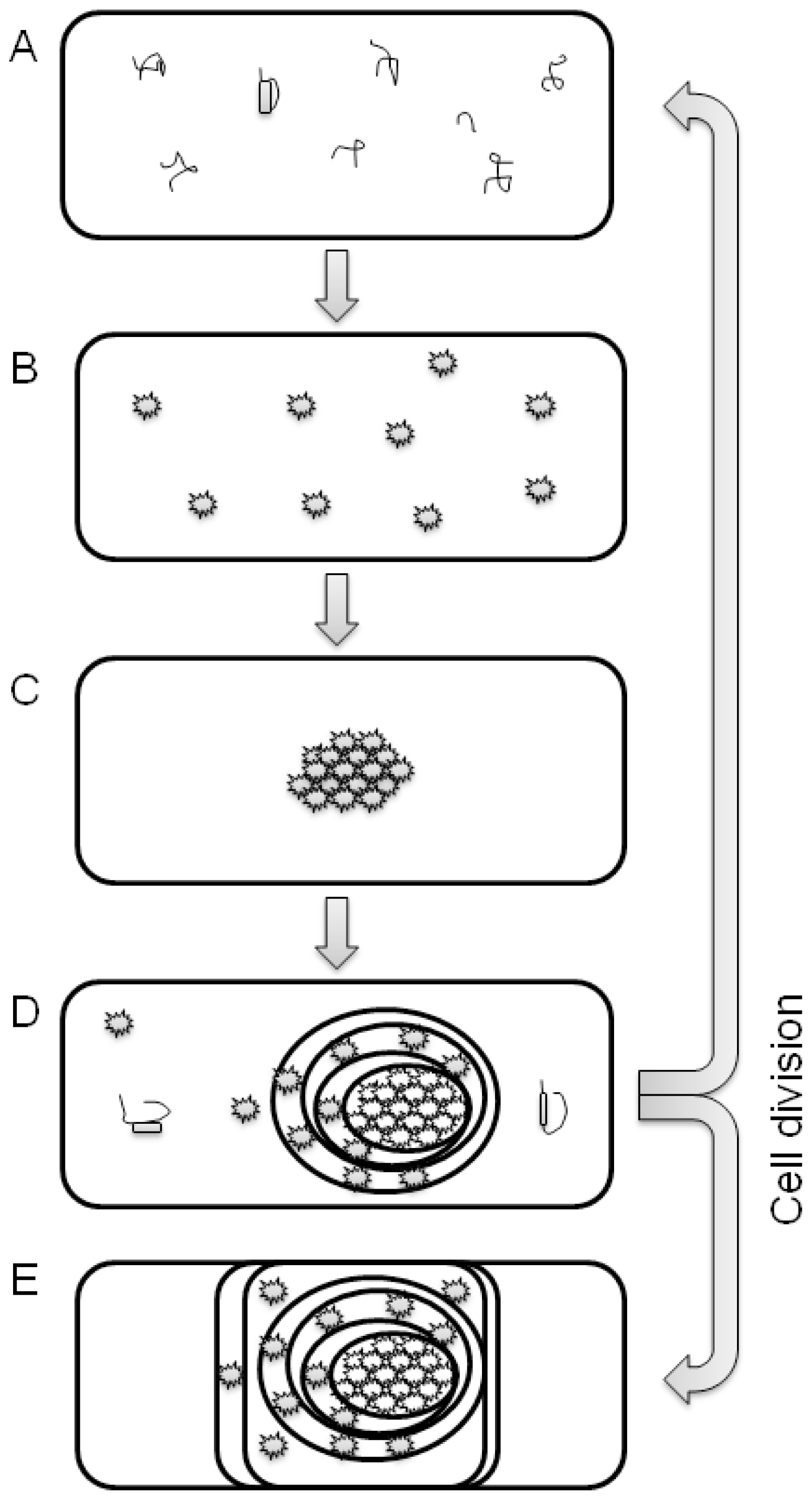
:1. Introduction
2. E. coli and Recombinant Protein Production
3. Protein Aggregation and IBs Formation
3. Properties of Properly Folded Protein Aggregates
4. What Can We Learn from Properly Folded Protein Aggregates and How Can We Use Them?
5. Conclusion and Perspectives
Acknowledgments
References
- Swartz, J.R. Advances in Escherichia coli production of therapeutic proteins. Curr. Opin. Biotechnol 2001, 12, 195–201. [Google Scholar]
- Graumann, K.; Premstaller, A. Manufacturing of recombinant therapeutic proteins in microbial systems. Biotechnol. J 2006, 1, 164–186. [Google Scholar]
- Schmidt, F.R. Recombinant expression systems in the pharmaceutical industry. Appl. Microbiol. Biotechnol 2004, 65, 363–372. [Google Scholar]
- Ferrer-Miralles, N.; Domingo-Espin, J.; Corchero, J.L.; Vazquez, E.; Villaverde, A. Microbial factories for recombinant pharmaceuticals. Microb. Cell Fact 2009, 8. [Google Scholar] [CrossRef]
- Walsh, G. Biopharmaceutical benchmarks 2006. Nat. Biotechnol 2006, 24, 769–776. [Google Scholar]
- de Groot, N.S.; Espargaro, A.; Morell, M.; Ventura, S. Studies on bacterial inclusion bodies. Future Microbiol 2008, 3, 423–435. [Google Scholar]
- Peternel, S.; Jevsevar, S.; Bele, M.; Gaberc-Porekar, V.; Menart, V. New properties of inclusion bodies with implications for biotechnology. Biotechnol. Appl. Biochem 2008, 49, 239–246. [Google Scholar]
- Peternel, S.; Grdadolnik, J.; Gaberc-Porekar, V.; Komel, R. Engineering inclusion bodies for non denaturing extraction of functional proteins. Microb. Cell Fact 2008, 7. [Google Scholar] [CrossRef]
- Garcia-Fruitos, E.; Gonzalez-Montalban, N.; Morell, M.; Vera, A.; Ferraz, R.M.; Aris, A.; Ventura, S.; Villaverde, A. Aggregation as bacterial inclusion bodies does not imply inactivation of enzymes and fluorescent proteins. Microb. Cell Fact 2005, 4. [Google Scholar] [CrossRef] [Green Version]
- de Groot, N.S.; Ventura, S. Effect of temperature on protein quality in bacterial inclusion bodies. FEBS Lett 2006, 580, 6471–6476. [Google Scholar]
- Smith, A. Protein misfolding. Nature 2003, 426, 883–909. [Google Scholar]
- Baneyx, F. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol 1999, 10, 411–421. [Google Scholar]
- Jevsevar, S.; Gaberc-Porekar, V.; Fonda, I.; Podobnik, B.; Grdadolnik, J.; Menart, V. Production of nonclassical inclusion bodies from which correctly folded protein can be extracted. Biotechnol. Prog 2005, 21, 632–639. [Google Scholar]
- Peternel, S.; Grdadolnik, J.; Gaberc-Porekar, V.; Komel, R. Engineering inclusion bodies for non denaturing extraction of functional proteins. Microb. Cell Fact 2008, 7. [Google Scholar] [CrossRef]
- Peternel, S. Designing Non-Classical Inclusion Bodies. In Ribosomal Proteins and Protein Engineering: Design, Selection and Applications; Ortendhal, V., Salchow, H., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 1–20. [Google Scholar]
- Jevsevar, S.; Palcic, J.; Jalen, S.; Pavko, A. Influence of the media composition on behavior of the pET expression systems. Acta Chim. Slov 2007, 54, 360–365. [Google Scholar]
- Sorensen, H.P.; Mortensen, K.K. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb. Cell Fact 2005, 4. [Google Scholar] [CrossRef]
- Weickert, M.J.; Doherty, D.H.; Best, E.A.; Olins, P.O. Optimization of heterologous protein production in Escherichia coli. Curr. Opin. Biotechnol 1996, 7, 494–499. [Google Scholar]
- Garcia-Fruitos, E.; Aris, A.; Villaverde, A. Localization of functional polypeptides in bacterial inclusion bodies. Appl. Environ. Microbiol 2007, 73, 289–294. [Google Scholar]
- Vera, A.; Gonzalez-Montalban, N.; Aris, A.; Villaverde, A. The conformational quality of insoluble recombinant proteins is enhanced at low growth temperatures. Biotechnol. Bioeng 2007, 96, 1101–1106. [Google Scholar]
- Peternel, S.; Bele, M.; Gaberc-Porekar, V.; Komel, R. Contraction of inclusion bodies with implications in biotechnology. Acta Chim. Slov 2008, 55, 608–612. [Google Scholar]
- Yin, J.; Li, G.; Ren, X.; Herrler, G. Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J. Biotechnol 2007, 127, 335–347. [Google Scholar]
- Hoffmann, F.; Rinas, U. Stress induced by recombinant protein production in Escherichia coli. Adv. Biochem. Eng. Biotechnol 2004, 89, 73–92. [Google Scholar]
- Gasser, B.; Saloheimo, M.; Rinas, U.; Dragosits, M.; Rodriguez-Carmona, E.; Baumann, K.; Giuliani, M.; Parrilli, E.; Branduardi, P.; Lang, C.; et al. Protein folding and conformational stress in microbial cells producing recombinant proteins: A host comparative overview. Microb. Cell Fact 2008, 7, 11. [Google Scholar]
- Garcia-Fruitos, E.; Sabate, R.; de Groot, N.S.; Villaverde, A.; Ventura, S. Biological role of bacterial inclusion bodies: a model for amyloid aggregation. FEBS J 2011, 278, 2419–2427. [Google Scholar]
- Carrio, M.M.; Villaverde, A. Protein aggregation as bacterial inclusion bodies is reversible. FEBS Lett 2001, 489, 29–33. [Google Scholar]
- Carrio, M.M.; Villaverde, A. Construction and deconstruction of bacterial inclusion bodies. J. Biotechnol 2002, 96, 3–12. [Google Scholar]
- De Marco, A.; Deuerling, E.; Mogk, A.; Tomoyasu, T.; Bukau, B. Chaperone-based procedure to increase yields of soluble recombinant proteins produced in E. coli. BMC Biotechnol 2007, 7. [Google Scholar] [CrossRef]
- Schrodel, A.; Volz, J.; De Marco, A. Fusion tags and chaperone co-expression modulate both the solubility and the inclusion body features of the recombinant CLIPB14 serine protease. J. Biotechnol 2005, 120, 2–10. [Google Scholar]
- Schrodel, A.; De Marco, A. Characterization of the aggregates formed during recombinant protein expression in bacteria. BMC Biochem 2005, 6. [Google Scholar] [CrossRef] [Green Version]
- Carrio, M.M.; Cubarsi, R.; Villaverde, A. Fine architecture of bacterial inclusion bodies. FEBS Lett 2000, 471, 7–11. [Google Scholar]
- de Groot, N.S.; Sabate, R.; Ventura, S. Amyloids in bacterial inclusion bodies. Trends Biochem. Sci 2009, 34, 408–416. [Google Scholar]
- Wickner, R.B.; Edskes, H.K.; Kryndushkin, D.; McGlinchey, R.; Bateman, D.; Kelly, A. Prion diseases of yeast: Amyloid structure and biology. Semin. Cell Dev. Biol 2011, 22, 469–475. [Google Scholar]
- Wickner, R.B.; Edskes, H.K.; Shewmaker, F.; Nakayashiki, T. Prions of fungi: Inherited structures and biological roles. Nat. Rev. Microbiol 2007, 5, 611–618. [Google Scholar]
- Villar-Pique, A.; Sabate, R.; Lopera, O.; Gibert, J.; Torne, J.M.; Santos, M.; Ventura, S. Amyloidlike protein inclusions in tobacco transgenic plants. PLoS One 2010, 5, e13625. [Google Scholar]
- Soto, C. Protein misfolding and disease; protein refolding and therapy. FEBS Lett 2001, 498, 204–207. [Google Scholar]
- Rokney, A.; Shagan, M.; Kessel, M.; Smith, Y.; Rosenshine, I.; Oppenheim, A.B. E. coli transports aggregated proteins to the poles by a specific and energy-dependent process. J. Mol. Biol 2009, 392, 589–601. [Google Scholar]
- Wang, L.; Maji, S.K.; Sawaya, M.R.; Eisenberg, D.; Riek, R. Bacterial inclusion bodies contain amyloid-like structure. PLoS Biol 2008, 6, e195. [Google Scholar]
- Garcia-Fruitos, E.; Rodriguez-Carmona, E.; Díez-Gil, C.; Ferraz, R.M.; Vazquez, E.; Corchero, J.L.; Cano-Sarabia, M.; Ratera, I.; Ventosa, N.; Veciana, J.; et al. Surface cell growth engineering assisted by a novel bacterial nanomaterial. Adv. Mat 2009, 21, 4249–4253. [Google Scholar]
- Peternel, S.; Komel, R. Isolation of biologically active nanomaterial (inclusion bodies) from bacterial cells. Microb. Cell Fact 2010, 9. [Google Scholar] [CrossRef]
- Bowden, G.A.; Paredes, A.M.; Georgiou, G. Structure and morphology of protein inclusion bodies in Escherichia coli. Biotechnology 1991, 9, 725–730. [Google Scholar]
- Garcia-Fruitos, E.; Seras-Franzoso, J.; Vazquez, E.; Villaverde, A. Tunable geometry of bacterial inclusion bodies as substrate materials for tissue engineering. Nanotechnology 2010, 21, 205101. [Google Scholar]
- Peternel, S. Bacterial cell disruption: A critical step in protein production. 2011. [Google Scholar]
- Villaverde, A.; Carrio, M.M. Protein aggregation in recombinant bacteria: Biological role of inclusion bodies. Biotechnol. Lett 2003, 25, 1385–1395. [Google Scholar]
- Peternel, S.; Gaberc-Porekar, V.; Komel, R. Bacterial growth conditions affect quality of GFP Expressed inside inclusion bodies. Acta Chim. Slov 2009, 56, 860–867. [Google Scholar]
- Carvajal, P.; Gibert, J.; Campos, N.; Lopera, O.; Barbera, E.; Torne, J.M.; Santos, M. Activity of maize transglutaminase overexpressed in Escherichia coli inclusion bodies: An alternative to protein refolding. Biotechnol. Prog 2011, 27, 232–240. [Google Scholar]
- Peternel, S.; Liovic, M. Production of recombinant proteins in bacteria: The inclusion bodies formation and their use in biomedicine. Recent Patents Biomed. Eng 2010, 3, 153–161. [Google Scholar]
- Plucienniczak, A.; Kesik, M.; Porebska, A.; Wedrychowicz, H.; Jedlina-Panasiuk, L. Inclusion bodies for the oral vaccination of animals. Eur. patent 20030789657, 3 December 2008. [Google Scholar]
- Plucienniczak, A.; Saczynska, V.; Porebska, A.; Szewczyk, B.; Ficinska, J. Inclusion bodies as antigens for the oral vaccination of animals. Eur. patent 1603938 A2, 14 December 2004. [Google Scholar]
- Kuzyk, M.A.; Kay, W.W.; Thornton, J.C.; Burian, J. Vaccines and agents for inducing immunity in fish against rickettsial diseases, and associated preventative therapy. Can. patent 2339327 A1, 15 March 2002. [Google Scholar]
- Levine, M. Mutants of lysine decarboxylase, vaccines for periodontitis, and methods of use. Eur. patent 2222693 A1, 1 September 2010. [Google Scholar]
- Royt, P. Use of pseudan and pseudan inclusion bodies. U.S. patent 2007/0099889 A1, 10 July 2006. [Google Scholar]
- Diez-Gil, C.; Krabbenborg, S.; Garcia-Fruitos, E.; Vazquez, E.; Rodriguez-Carmona, E.; Ratera, I.; Ventosa, N.; Seras-Franzoso, J.; Cano-Garrido, O.; Ferrer-Miralles, N.; et al. The nanoscale properties of bacterial inclusion bodies and their effect on mammalian cell proliferation. Biomaterials 2010, 31, 5805–5812. [Google Scholar]
- Nahalka, J.; Nidetzky, B. Fusion to a pull-down domain: a novel approach of producing Trigonopsis variabilisD-amino acid oxidase as insoluble enzyme aggregates. Biotechnol. Bioeng 2007, 97, 454–461. [Google Scholar]
- Nahalka, J.; Gemeiner, P.; Bucko, M.; Wang, P.G. Bioenergy beads: A tool for regeneration of ATP/NTP in biocatalytic synthesis. Artif. Cells Blood Substit. Biotechnol 2006, 34, 515–521. [Google Scholar]
- Nahalka, J. Physiological aggregation of maltodextrin phosphorylase from Pyrococcus furiosus and its application in a process of batch starch degradation to alpha-D-glucose-1-phosphate. J. Ind. Microbiol. Biotechnol 2008, 35, 219–223. [Google Scholar]
- Nahalka, J.; Patoprsty, V. Enzymatic synthesis of sialylation substrates powered by a novel polyphosphate kinase (PPK3). Org. Biomol. Chem 2009, 7, 1778–1780. [Google Scholar]
- Nahalka, J.; Vikartovska, A.; Hrabarova, E. A crosslinked inclusion body process for sialic acid synthesis. J. Biotechnol 2008, 134, 146–153. [Google Scholar]
- Nahalka, J.; Dib, I.; Nidetzky, B. Encapsulation of Trigonopsis variabilis D-amino acid oxidase and fast comparison of the operational stabilities of free and immobilized preparations of the enzyme. Biotechnol. Bioeng 2008, 99, 251–260. [Google Scholar]
- Nahalka, J.; Mislovicova, D.; Kavcova, H. Targeting lectin activity into inclusion bodies for the characterisation of glycoproteins. Mol. Biosyst 2009, 5, 819–821. [Google Scholar]
- Garcia-Fruitos, E. Inclusion bodies: A new concept. Microb. Cell Fact 2010, 9, 80. [Google Scholar]
- Garcia-Fruitos, E.; Vazquez, E.; Diez-Gil, C.; Corchero, J.L.; Seras-Franzoso, J.; Ratera, I.; Veciana, J.; Villaverde, A. Bacterial inclusion bodies: Making gold from waste. 2011. [Google Scholar]




© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peternel, Š.; Komel, R. Active Protein Aggregates Produced in Escherichia coli. Int. J. Mol. Sci. 2011, 12, 8275-8287. https://doi.org/10.3390/ijms12118275
Peternel Š, Komel R. Active Protein Aggregates Produced in Escherichia coli. International Journal of Molecular Sciences. 2011; 12(11):8275-8287. https://doi.org/10.3390/ijms12118275
Chicago/Turabian StylePeternel, Špela, and Radovan Komel. 2011. "Active Protein Aggregates Produced in Escherichia coli" International Journal of Molecular Sciences 12, no. 11: 8275-8287. https://doi.org/10.3390/ijms12118275



