Physicochemical Properties of Glycine-Based Ionic Liquid [QuatGly-OEt][EtOSO3] (2-Ethoxy-1-ethyl-1,1-dimethyl-2-oxoethanaminium ethyl sulfate) and Its Binary Mixtures with Poly(ethylene glycol) (Mw = 200) at Various Temperatures
Abstract
:1. Introduction
2. Results and Discussion
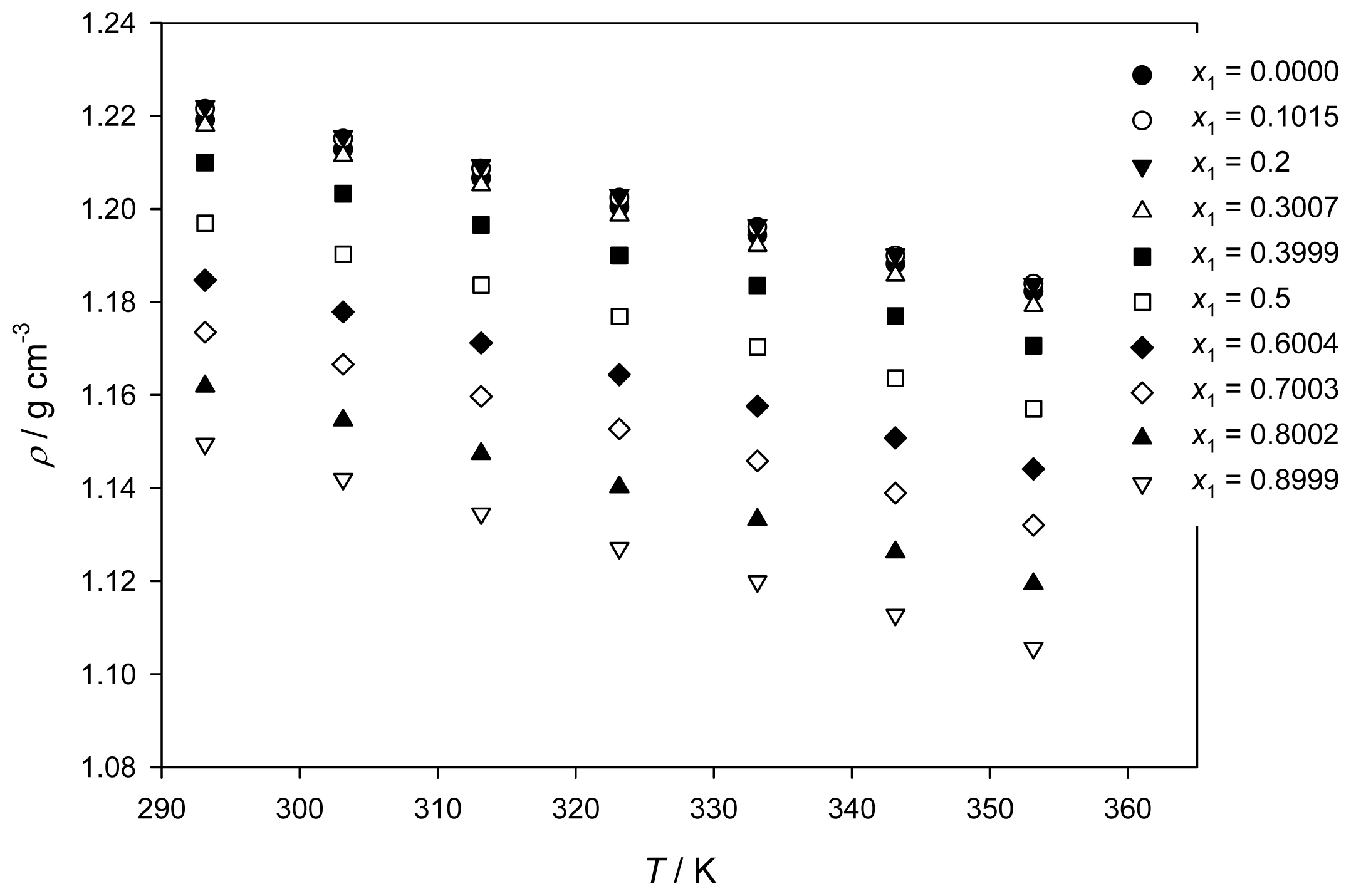
2.1. Density and Excess Molar Volume
2.2. Volume Expansivity and Excess Volume Expansivity
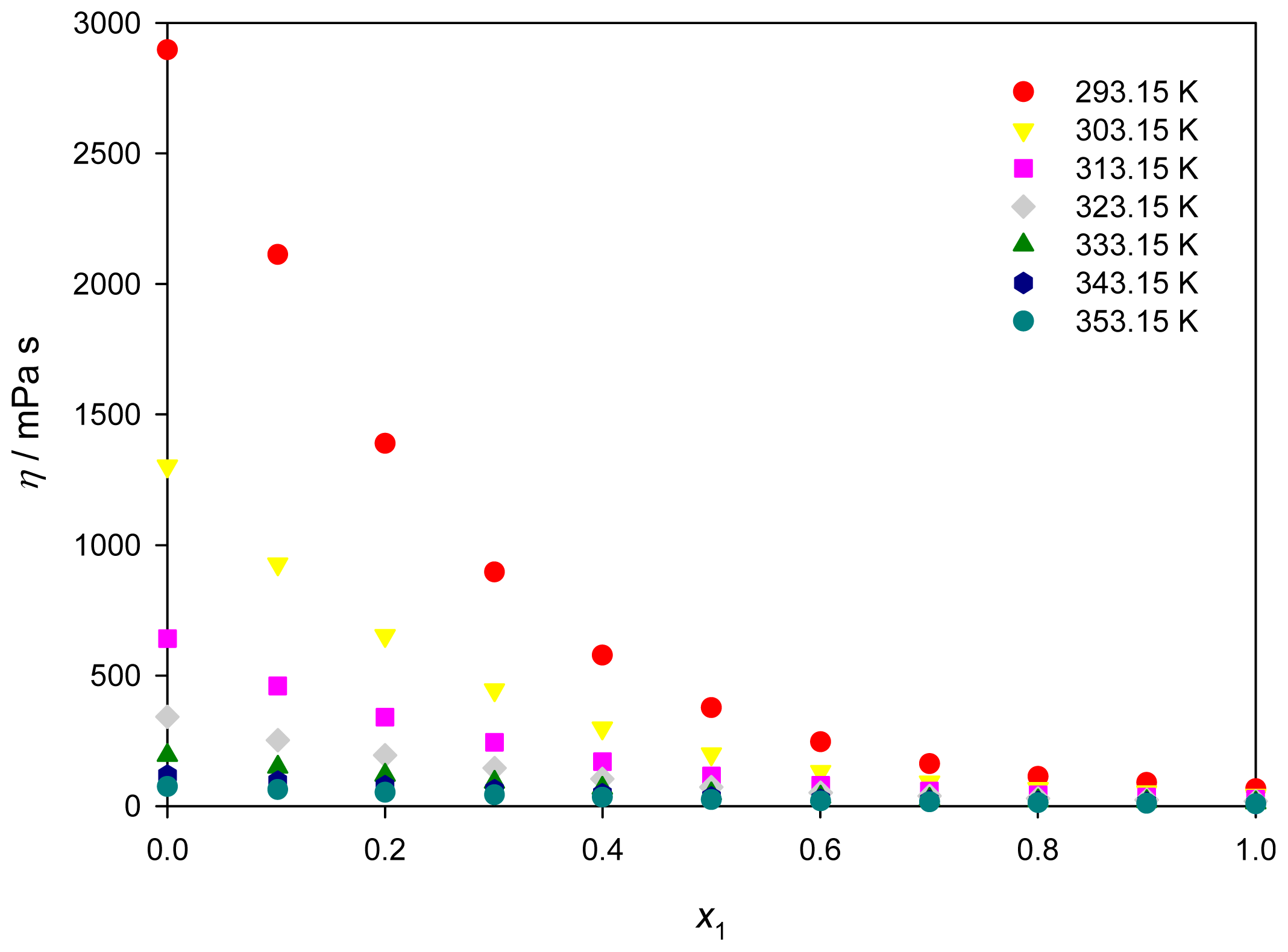
2.3. Viscosity
2.4. Refractive Index Deviations and Molar Refraction Deviations
2.5. Conductivity
2.6. Redlich-Kister Equation for Binary System
3. Experimental Section
3.1. Materials
3.2. 2-Ethoxy-1-ethyl-1,1-dimethyl-2-oxoethanaminium ethyl Sulfate ([QuatGly-OEt][EtOSO3])
3.3. Measurements
4. Conclusions
Acknowledgements
References
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; VCH: Weinheim, Germany, 2003. [Google Scholar]
- Domańska, U. Physico-chemical properties and phase behaviour of pyrrolidinium-based ionic liquids. Int. J. Mol. Sci 2010, 11, 1825–1841. [Google Scholar]
- Wu, T.Y.; Su, S.G.; Wang, H.P.; Lin, Y.C.; Gung, S.T.; Lin, M.W.; Sun, I.W. Electrochemical studies and self diffusion coefficients in cyclic ammonium based ionic liquids with allyl substituents. Electrochim. Acta 2011, 56, 3209–3218. [Google Scholar]
- Wu, T.Y.; Su, S.G.; Wang, H.P.; Sun, I.W. Glycine-based ionic liquids as potential electrolyte for electrochemical studies of organometallic and organic redox couples. Electrochem. Commun 2011, 13, 237–241. [Google Scholar]
- Wu, T.Y.; Su, S.G.; Lin, K.F.; Lin, Y.C.; Wang, H.P.; Lin, M.W.; Gung, S.T.; Sun, I.W. Voltammetric and physicochemical characterization of hydroxyl- and ether-functionalized onium bis(trifluoromethanesulfonyl)imide ionic liquids. Electrochim. Acta 2011, 56, 7278–7287. [Google Scholar]
- Soriano, A.N.; Doma, B.T.; Li, M.H. Density and refractive index measurements of 1-ethyl-3-methylimidazolium-based ionic liquids. J. Taiwan Inst. Chem. Eng 2010, 41, 115–121. [Google Scholar]
- Wu, T.Y.; Sun, I.W.; Gung, S.T.; Lin, M.W.; Chen, B.K.; Wang, H.P.; Su, S.G. Effects of cations and anions on transport properties in tetrafluoroborate-based ionic liquids. J. Taiwan Inst. Chem. Eng 2011, 42, 513–522. [Google Scholar]
- Lu, D.; Shomali, N.; Shen, A. Task specific ionic liquid for direct electrochemistry of metal oxides. Electrochem. Commun 2010, 12, 1214–1217. [Google Scholar]
- Louros, C.L.S.; Cláudio, A.F.M.; Neves, C.M.S.S.; Freire, M.G.; Marrucho, I.M.; Pauly, J.; Coutinho, J.A.P. Extraction of biomolecules using phosphonium-based ionic liquids + K3PO4 aqueous biphasic systems. Int. J. Mol. Sci 2010, 11, 1777–1791. [Google Scholar]
- Li, S.; Tian, M.; Row, K.H. Effect of mobile phase additives on the resolution of four bioactive compounds by RP-HPLC. Int. J. Mol. Sci 2010, 11, 2229–2240. [Google Scholar]
- Zhao, F.; Xiao, F.; Zeng, B. Electrodeposition of PtCo alloy nanoparticles on inclusion complex film of functionalized cyclodextrin-ionic liquid and their application in glucose sensing. Electrochem. Commun 2010, 12, 168–171. [Google Scholar]
- Subramaniam, P.; Mohamad, S.; Alias, Y. Synthesis and characterization of the inclusion complex of dicationic ionic liquid and β-cyclodextrin. Int. J. Mol. Sci 2010, 11, 3675–3685. [Google Scholar]
- Wu, T.Y.; Tsao, M.H.; Chen, F.L.; Su, S.G.; Chang, C.W.; Wang, H.P.; Lin, Y.C.; Sun, I.W. Synthesis and characterization of three organic dyes with various donors and rhodanine ring acceptor for use in dye-sensitized solar cells. J. Iran. Chem. Soc 2010, 7, 707–720. [Google Scholar]
- Tsao, M.H.; Wu, T.Y.; Wang, H.P.; Sun, I.W.; Su, S.G.; Lin, Y.C.; Chang, C.W. An efficient metal free sensitizer for dye-sensitized solar cells. Mater. Lett 2011, 65, 583–586. [Google Scholar]
- Wu, T.Y.; Tsao, M.H.; Chen, F.L.; Su, S.G.; Chang, C.W.; Wang, H.P.; Lin, Y.C.; Ou-Yang, W.C.; Sun, I.W. Synthesis and characterization of organic dyes containing various donors and acceptors. Int. J. Mol. Sci 2010, 11, 329–353. [Google Scholar]
- Li, Z.H.; Xia, Q.L.; Liu, L.L.; Lei, G.T.; Xiao, Q.Z.; Gao, D.S.; Zhou, X.D. Effect of zwitterionic salt on the electrochemical properties of a solid polymer electrolyte with high temperature stability for lithium ion batteries. Electrochim. Acta 2010, 56, 804–809. [Google Scholar]
- Sun, X.G.; Dai, S. Electrochemical investigations of ionic liquids with vinylene carbonate for applications in rechargeable lithium ion batteries. Electrochim. Acta 2010, 55, 4618–4626. [Google Scholar]
- Che, Q.; He, R.; Yang, J.; Feng, L.; Savinell, R.F. Phosphoric acid doped high temperature proton exchange membranes based on sulfonated polyetheretherketone incorporated with ionic liquids. Electrochem. Commun 2010, 12, 647–649. [Google Scholar]
- Lakshminarayana, G.; Nogami, M. Inorganic-organic hybrid membranes with anhydrous proton conduction prepared from tetramethoxysilane/methyl-tri methoxysilane/trimethylphosphate and 1-ethyl-3-methylimidazolium-bis(trifluoro methanesulfonyl) imide for H2/O2 fuel cells. Electrochim. Acta 2010, 55, 1160–1168. [Google Scholar]
- Wu, T.Y.; Su, S.G.; Gung, S.T.; Lin, M.W.; Lin, Y.C.; Ou-Yang, W.C.; Sun, I.W.; Lai, C.A. Synthesis and characterization of protic ionic liquids containing cyclic amine cations and tetrafluoroborate anion. J. Iran. Chem. Soc 2011, 8, 149–165. [Google Scholar]
- Wu, T.Y.; Su, S.G.; Gung, S.T.; Lin, M.W.; Lin, Y.C.; Lai, C.A.; Sun, I.W. Ionic liquids containing an alkyl sulfate group as potential electrolytes. Electrochim. Acta 2010, 55, 4475–4482. [Google Scholar]
- Soriano, A.N.; Agapito, A.M.; Lagumbay, L.J.L.I.; Caparanga, A.R.; Li, M.H. A simple approach to predict molar heat capacity of ionic liquids using group-additivity method. J. Taiwan Inst. Chem. Eng 2010, 41, 314. [Google Scholar]
- Kagimoto, J.; Taguchi, S.; Fukumoto, K.; Ohno, H. Hydrophobic and low-density amino acid ionic liquids. J. Mol. Liq 2010, 153, 133–138. [Google Scholar]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room Temperature Ionic Liquids from 20 Natural Amino Acids. J. Am. Chem. Soc 2005, 127, 2398–2399. [Google Scholar]
- Kagimoto, J.; Fukumoto, K.; Ohno, H. Effect of tetrabutylphosphonium cation on the physico-chemical properties of amino-acid ionic liquids. Chem. Commun 2006, 2254–2256. [Google Scholar]
- Tao, G.H.; He, L.; Sun, N.; Kou, Y. New generation ionic liquids: Cations derived from amino acids. Chem. Commun 2005, 3562–3564. [Google Scholar]
- Fukumoto, K.; Ohno, H. Design and synthesis of hydrophobic and chiral anions from amino acids as precursor for functional ionic liquids. Chem. Commun 2006, 3081–3083. [Google Scholar]
- Wu, T.Y.; Wang, H.C.; Su, S.G.; Gung, S.T.; Lin, M.W.; Lin, C.B. Characterization of ionic conductivity, viscosity, density, and self-diffusion coefficient for binary mixtures of polyethyleneglycol (or polyethyleneimine) organic solvent with room temperature ionic liquid BMIBF4 (or BMIPF6). J. Taiwan Inst. Chem. Eng 2010, 41, 315–325. [Google Scholar]
- Li, X.; Hou, M.; Zhang, Z.; Han, B.; Yang, G.; Wang, X.; Zou, L. Absorption of CO2 by ionic liquid/polyethylene glycol mixture and the thermodynamic parameters. Green Chem 2008, 10, 879–884. [Google Scholar]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem 2005, 7, 64–82. [Google Scholar]
- Sarkar, A.; Trivedi, S.; Pandey, S. Polymer molecular weight-dependent unusual fluorescence probe behavior within 1-butyl-3-methylimidazolium hexafluorophosphate + poly(ethylene glycol). J. Phys. Chem. B 2009, 113, 7606–7614. [Google Scholar]
- Thirumurugan, A. Use of ionic liquids in synthesis of nanocrystals, nanorods and nanowires of elemental chalcogens. Bull. Mater. Sci 2007, 30, 179–182. [Google Scholar]
- Zafarani-Moattar, M.T.; Shekaari, H. Volumetric and speed of sound of ionic liquid, 1-butyl-3-methylimidazolium hexafluorophosphate with acetonitrile and methanol at T = (298.15 to 318.15) K. J. Chem. Eng. Data 2005, 50, 1694–1699. [Google Scholar]
- Tamura, K.; Nakamura, M.; Murakami, S. Excess volumes of water + acetonitrile and water + dimethylsulfoxide at 30 °C and the effect of the excess thermal expansivity coefficients on derived thermodynamic properties. J. Solution Chem 1997, 26, 1199–1207. [Google Scholar]
- Hwang, K.S.; Park, S.W.; Park, D.W.; Oh, K.J.; Kim, S.S. Absorption of carbon dioxide into diisopropanolamine solutions of polar organic solvents. J. Taiwan Inst. Chem. Eng 2010, 41, 16–21. [Google Scholar]
- Yu, Z.; Zhang, Q.; Qin, D.; Luo, Y.; Li, D.; Shen, Q.; Toyoda, T.; Meng, Q. Highly efficient quasi-solid-state quantum-dot-sensitized solar cell based on hydrogel electrolytes. Electrochem. Commun 2010, 12, 1776–1779. [Google Scholar]
- Wu, T.Y.; Su, S.G.; Lin, Y.C.; Lin, M.W.; Gung, S.T.; Sun, I.W. Electrochemical and physicochemical properties of cyclic amine-based Brønsted acidic ionic liquids. Electrochim. Acta 2010, 56, 853–862. [Google Scholar]
- Rodriguez, H.; Brennecke, J.F. Temperature and composition dependence of the density and viscosity of binary mixtures of water + ionic liquid. J. Chem. Eng. Data 2006, 51, 2145–2155. [Google Scholar]
- Tian, Y.; Wang, X.; Wang, J. Densities and viscosities of 1-butyl-3-methylimidazolium tetrafluoroborate + molecular solvent binary mixtures. J. Chem. Eng. Data 2008, 53, 2056–2059. [Google Scholar]
- Wu, T.Y.; Wang, H.C.; Su, S.G.; Gung, S.T.; Lin, M.W.; Lin, C.B. Aggregation influence of polyethyleneglycol organic solvents with ionic liquids BMIBF4 and BMIPF6. J. Chin. Chem. Soc 2010, 57, 44–55. [Google Scholar]
- Wu, T.Y.; Chen, B.K.; Hao, L.; Peng, Y.C.; Sun, I.W. Effect of temperature on the physico-chemical properties of a room temperature ionic liquid (1-methyl-3-pentylimidazolium hexafluorophosphate) with polyethylene glycol oligomer. Int. J. Mol. Sci 2011, 12, 2598–2617. [Google Scholar]
- Eyring, H.; John, M.S. Significant Liquid Structures; John Wiley & Sons: New York, NY, USA, 1969. [Google Scholar]
- Fermeglia, M.; Torriano, G. Density, viscosity, and refractive index for binary systems of n-C16 and four nonlinear alkanes at 298.15 K. J. Chem. Eng. Data 1999, 44, 965–969. [Google Scholar]
- Arce, A.; Rodil, E.; Soto, A. Molar volume, molar refraction, and isentropic compressibility changes of mixing at 25 °C for the system ethanol + methanol + dibutyl ether. J. Sol. Chem 1998, 27, 911–923. [Google Scholar]
- Moumouzias, G.; Ritzoulis, G. Densities, relative permittivities, and refractive indices of the binary systems propylene carbonate + o-xylene and propylene carbonate + m-xylene at (15, 20, 25, 30, and 35) °C. J. Chem. Eng. Data 2000, 45, 202–206. [Google Scholar]
- Hawrylak, B.; Andrecyk, S.; Gabriel, C.E.; Gracie, K.; Palepu, R. Viscosity, surface tension, and refractive index measurements of mixtures of isomeric butanediols with water. J. Sol. Chem 1998, 27, 827–841. [Google Scholar]
- Moumouzias, G.; Ritzoulis, G. Relative permittivities and refractive indices of γ-butyrolactone with o-xylene and m-xylene. J. Chem. Eng. Data 1999, 44, 1273–1278. [Google Scholar]
- Every, H.; Bishop, A.G.; Forsyth, M.; MacFarlane, D.R. Ion diffusion in molten salt mixtures. Electrochim. Acta 2000, 45, 1279–1284. [Google Scholar]
- Zhao, Y.; Gao, S.J.; Wang, J.J.; Tang, J.M. Aggregation of ionic liquids CnmimBr (n = 4, 6, 8, 10, 12) in D2O: A NMR study. J. Phys. Chem. B 2008, 112, 2031–2039. [Google Scholar]
- Redlich, O.; Kister, A.T. Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem 1948, 40, 345–348. [Google Scholar]
- Paul, A.; Kumar, P.; Samanta, A. On the optical properties of the imidazolium ionic liquids. J. Phys. Chem. B 2005, 109, 9148–9153. [Google Scholar]







| T/K
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x1 | 293.15 | 298.15 | 303.15 | 308.15 | 313.15 | 318.15 | 323.15 | 328.15 | 333.15 | 338.15 | 343.15 | 348.15 | 353.15 |
| ρ/g cm−3 | |||||||||||||
| 0 | 1.2191 | 1.2160 | 1.2128 | 1.2097 | 1.2066 | 1.2035 | 1.2004 | 1.1973 | 1.1943 | 1.1913 | 1.1882 | 1.1852 | 1.1822 |
| 0.1015 | 1.2215 | 1.2183 | 1.2151 | 1.2119 | 1.2087 | 1.2055 | 1.2024 | 1.1993 | 1.1961 | 1.1930 | 1.1900 | 1.1869 | 1.1839 |
| 0.2000 | 1.2221 | 1.2189 | 1.2157 | 1.2125 | 1.2094 | 1.2062 | 1.2030 | 1.1998 | 1.1966 | 1.1934 | 1.1902 | 1.1871 | 1.1839 |
| 0.3007 | 1.2180 | 1.2148 | 1.2115 | 1.2083 | 1.2051 | 1.2018 | 1.1986 | 1.1954 | 1.1921 | 1.1889 | 1.1857 | 1.1824 | 1.1792 |
| 0.3999 | 1.2100 | 1.2066 | 1.2033 | 1.1999 | 1.1966 | 1.1933 | 1.1900 | 1.1867 | 1.1835 | 1.1802 | 1.1770 | 1.1738 | 1.1706 |
| 0.5000 | 1.1969 | 1.1935 | 1.1902 | 1.1869 | 1.1836 | 1.1802 | 1.1769 | 1.1736 | 1.1703 | 1.1669 | 1.1636 | 1.1603 | 1.1570 |
| 0.6004 | 1.1847 | 1.1813 | 1.1779 | 1.1746 | 1.1712 | 1.1678 | 1.1644 | 1.1610 | 1.1576 | 1.1542 | 1.1508 | 1.1475 | 1.1441 |
| 0.7003 | 1.1735 | 1.1700 | 1.1666 | 1.1631 | 1.1597 | 1.1562 | 1.1527 | 1.1493 | 1.1458 | 1.1424 | 1.1389 | 1.1355 | 1.1320 |
| 0.8002 | 1.1619 | 1.1582 | 1.1546 | 1.1510 | 1.1474 | 1.1438 | 1.1402 | 1.1367 | 1.1332 | 1.1297 | 1.1262 | 1.1228 | 1.1194 |
| 0.8999 | 1.1495 | 1.1457 | 1.1419 | 1.1382 | 1.1345 | 1.1308 | 1.1271 | 1.1235 | 1.1199 | 1.1163 | 1.1127 | 1.1092 | 1.1056 |
| 1 | 1.1312 | 1.1273 | 1.1234 | 1.1195 | 1.1157 | 1.1119 | 1.1081 | 1.1044 | 1.1007 | 1.0970 | 1.0933 | 1.0896 | 1.0860 |
| VmE /cm3 mol−1 | |||||||||||||
| 0 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| 0.1015 | −1.7372 | −1.7458 | −1.7543 | −1.7629 | −1.7714 | −1.7799 | −1.7885 | −1.7970 | −1.8056 | −1.8141 | −1.8226 | −1.8312 | −1.8397 |
| 0.2000 | −3.0868 | −3.1224 | −3.1551 | −3.1847 | −3.2112 | −3.2347 | −3.2551 | −3.2723 | −3.2864 | −3.2974 | −3.3051 | −3.3096 | −3.3108 |
| 0.3007 | −3.6416 | −3.6832 | −3.7219 | −3.7574 | −3.7899 | −3.8193 | −3.8455 | −3.8686 | −3.8885 | −3.9051 | −3.9186 | −3.9287 | −3.9356 |
| 0.3999 | −3.5474 | −3.5758 | −3.6042 | −3.6326 | −3.6610 | −3.6895 | −3.7179 | −3.7463 | −3.7747 | −3.8031 | −3.8315 | −3.8599 | −3.8883 |
| 0.5000 | −2.6761 | −2.7217 | −2.7640 | −2.8032 | −2.8392 | −2.8719 | −2.9014 | −2.9276 | −2.9505 | −2.9700 | −2.9861 | −2.9988 | −3.0081 |
| 0.6004 | −2.0795 | −2.1240 | −2.1653 | −2.2032 | −2.2379 | −2.2692 | −2.2971 | −2.3216 | −2.3427 | −2.3603 | −2.3745 | −2.3851 | −2.3922 |
| 0.7003 | −1.7384 | −1.7815 | −1.8213 | −1.8577 | −1.8906 | −1.9201 | −1.9461 | −1.9685 | −1.9874 | −2.0027 | −2.0144 | −2.0225 | −2.0268 |
| 0.8002 | −1.4389 | −1.4592 | −1.4795 | −1.4998 | −1.5202 | −1.5405 | −1.5608 | −1.5811 | −1.6014 | −1.6217 | −1.6420 | −1.6623 | −1.6826 |
| 0.8999 | −1.1124 | −1.1252 | −1.1380 | −1.1508 | −1.1636 | −1.1764 | −1.1892 | −1.2020 | −1.2148 | −1.2276 | −1.2404 | −1.2532 | −1.2660 |
| 1 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| x1 | b0 | 104 b1 | 107 b2 | 104 σ |
|---|---|---|---|---|
| 0 | 1.432 | −8.2 | 3.15 | 1.6 |
| 0.1015 | 1.439 | −8.4 | 3.27 | 3.6 |
| 0.2000 | 1.409 | −6.4 | 0 | 1.7 |
| 0.3007 | 1.408 | −6.5 | 0 | 3.2 |
| 0.3999 | 1.44 | −8.9 | 3.62 | 2.0 |
| 0.5000 | 1.392 | −6.7 | 0 | 1.9 |
| 0.6004 | 1.383 | −6.8 | 0 | 1.7 |
| 0.7003 | 1.376 | −6.9 | 0 | 3.6 |
| 0.8002 | 1.415 | −9.9 | 4.41 | 4.1 |
| 0.8999 | 1.413 | −10.4 | 4.74 | 7.1 |
| 1 | 1.405 | −10.8 | 5.11 | 12.2 |
| x1 | T/K
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 293.15 | 298.15 | 303.15 | 308.15 | 313.15 | 318.15 | 323.15 | 328.15 | 333.15 | 338.15 | 343.15 | 348.15 | 353.15 | |
| 104 α/K−1 | |||||||||||||
| 0 | 5.0413 | 5.0544 | 5.0675 | 5.0807 | 5.0938 | 5.1069 | 5.1200 | 5.1331 | 5.1462 | 5.1593 | 5.1724 | 5.1855 | 5.1986 |
| 0.1015 | 5.1363 | 5.1499 | 5.1635 | 5.1771 | 5.1907 | 5.2044 | 5.2180 | 5.2316 | 5.2452 | 5.2588 | 5.2724 | 5.2860 | 5.2996 |
| 0.2000 | 5.2124 | 5.2260 | 5.2397 | 5.2534 | 5.2673 | 5.2812 | 5.2952 | 5.3092 | 5.3233 | 5.3375 | 5.3518 | 5.3662 | 5.3806 |
| 0.3007 | 5.3144 | 5.3285 | 5.3428 | 5.3571 | 5.3715 | 5.3859 | 5.4005 | 5.4151 | 5.4298 | 5.4446 | 5.4594 | 5.4744 | 5.4894 |
| 0.3999 | 5.4207 | 5.4358 | 5.4510 | 5.4662 | 5.4814 | 5.4966 | 5.5118 | 5.5270 | 5.5422 | 5.5573 | 5.5725 | 5.5877 | 5.6029 |
| 0.5000 | 5.5578 | 5.5733 | 5.5889 | 5.6046 | 5.6203 | 5.6361 | 5.6521 | 5.6681 | 5.6842 | 5.7004 | 5.7167 | 5.7331 | 5.7496 |
| 0.6004 | 5.7170 | 5.7334 | 5.7499 | 5.7665 | 5.7831 | 5.7999 | 5.8168 | 5.8337 | 5.8508 | 5.8680 | 5.8852 | 5.9026 | 5.9201 |
| 0.7003 | 5.8952 | 5.9126 | 5.9301 | 5.9478 | 5.9655 | 5.9834 | 6.0013 | 6.0194 | 6.0376 | 6.0558 | 6.0742 | 6.0927 | 6.1114 |
| 0.8002 | 6.1003 | 6.1196 | 6.1389 | 6.1582 | 6.1776 | 6.1969 | 6.2162 | 6.2355 | 6.2548 | 6.2741 | 6.2935 | 6.3128 | 6.3321 |
| 0.8999 | 6.3560 | 6.3770 | 6.3980 | 6.4190 | 6.4400 | 6.4610 | 6.4820 | 6.5030 | 6.5240 | 6.5450 | 6.5660 | 6.5870 | 6.6080 |
| 1 | 6.6532 | 6.6763 | 6.6993 | 6.7224 | 6.7455 | 6.7685 | 6.7916 | 6.8146 | 6.8377 | 6.8607 | 6.8838 | 6.9069 | 6.9299 |
| 104 αE/K−1 | |||||||||||||
| 0 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| 0.1015 | −0.0318 | −0.0322 | −0.0325 | −0.0329 | −0.0333 | −0.0337 | −0.0340 | −0.0344 | −0.0348 | −0.0352 | −0.0356 | −0.0360 | −0.0363 |
| 0.2000 | −0.0850 | −0.0863 | −0.0875 | −0.0886 | −0.0896 | −0.0906 | −0.0915 | −0.0923 | −0.0930 | −0.0937 | −0.0943 | −0.0948 | −0.0953 |
| 0.3007 | −0.1221 | −0.1237 | −0.1253 | −0.1268 | −0.1282 | −0.1296 | −0.1308 | −0.1320 | −0.1331 | −0.1342 | −0.1351 | −0.1360 | −0.1368 |
| 0.3999 | −0.1604 | −0.1620 | −0.1635 | −0.1651 | −0.1667 | −0.1682 | −0.1698 | −0.1714 | −0.1729 | −0.1745 | −0.1761 | −0.1777 | −0.1793 |
| 0.5000 | −0.1771 | −0.1794 | −0.1815 | −0.1836 | −0.1856 | −0.1875 | −0.1893 | −0.1910 | −0.1926 | −0.1942 | −0.1956 | −0.1970 | −0.1983 |
| 0.6004 | −0.1812 | −0.1835 | −0.1858 | −0.1879 | −0.1900 | −0.1920 | −0.1939 | −0.1957 | −0.1974 | −0.1990 | −0.2004 | −0.2018 | −0.2031 |
| 0.7003 | −0.1751 | −0.1775 | −0.1797 | −0.1819 | −0.1839 | −0.1859 | −0.1877 | −0.1894 | −0.1911 | −0.1926 | −0.1940 | −0.1953 | −0.1965 |
| 0.8002 | −0.1524 | −0.1540 | −0.1555 | −0.1570 | −0.1585 | −0.1601 | −0.1616 | −0.1632 | −0.1647 | −0.1662 | −0.1678 | −0.1693 | −0.1709 |
| 0.8999 | −0.0904 | −0.0913 | −0.0922 | −0.0932 | −0.0941 | −0.0950 | −0.0960 | −0.0969 | −0.0979 | −0.0988 | −0.0997 | −0.1007 | −0.1016 |
| 1 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| x1 | T/K
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 293.15 | 298.15 | 303.15 | 308.15 | 313.15 | 318.15 | 323.15 | 328.15 | 333.15 | 338.15 | 343.15 | 348.15 | 353.15 | |
| η/mPa·s | |||||||||||||
| 0 | 2897.3 | 1917.3 | 1301.5 | 904.2 | 641.6 | 464.2 | 341.8 | 255.9 | 194.4 | 149.8 | 116.9 | 92.4 | 73.8 |
| 0.1015 | 2111.9 | 1374.7 | 926.5 | 644.0 | 460.0 | 336.7 | 251.9 | 192.1 | 149.2 | 117.7 | 94.2 | 76.4 | 62.7 |
| 0.2000 | 1389.2 | 937.5 | 652.0 | 465.9 | 341.0 | 255.0 | 194.4 | 150.8 | 118.9 | 95.1 | 77.0 | 63.2 | 52.4 |
| 0.3007 | 896.4 | 622.9 | 445.2 | 326.1 | 244.3 | 186.7 | 145.2 | 114.8 | 92.1 | 74.9 | 61.7 | 51.3 | 43.2 |
| 0.3999 | 577.5 | 409.6 | 298.4 | 222.6 | 169.6 | 131.7 | 104.0 | 83.4 | 67.9 | 55.9 | 46.6 | 39.2 | 33.3 |
| 0.5000 | 376.6 | 270.5 | 199.4 | 150.4 | 115.8 | 90.9 | 72.5 | 58.7 | 48.1 | 40.0 | 33.6 | 28.5 | 24.4 |
| 0.6004 | 246.3 | 178.1 | 132.6 | 101.2 | 79.0 | 62.8 | 50.9 | 41.8 | 34.9 | 29.4 | 25.1 | 21.6 | 18.8 |
| 0.7003 | 162.3 | 120.3 | 91.6 | 71.5 | 57.0 | 46.2 | 38.1 | 31.9 | 27.0 | 23.2 | 20.1 | 17.6 | 15.5 |
| 0.8002 | 112.9 | 85.8 | 66.8 | 53.0 | 42.8 | 35.1 | 29.2 | 24.7 | 21.0 | 18.1 | 15.7 | 13.8 | 12.2 |
| 0.8999 | 89.8 | 68.5 | 53.4 | 42.5 | 34.5 | 28.4 | 23.7 | 20.0 | 17.1 | 14.8 | 12.9 | 11.3 | 10.1 |
| 1 | 65.8 | 51.0 | 40.3 | 32.4 | 26.4 | 21.9 | 18.3 | 15.5 | 13.2 | 11.4 | 9.9 | 8.7 | 7.7 |
| Δη/mPa·s | |||||||||||||
| 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 0.1015 | −498.0 | −353.2 | −247.0 | −171.7 | −119.1 | −82.6 | −57.1 | −39.3 | −26.8 | −18.1 | −11.9 | −7.4 | −4.3 |
| 0.2000 | −941.7 | −606.6 | −397.3 | −264.0 | −177.6 | −120.7 | −82.7 | −57.0 | −39.3 | −27.1 | −18.5 | −12.5 | −8.2 |
| 0.3007 | −1149.5 | −733.3 | −477.2 | −316.0 | −212.3 | −144.5 | −99.3 | −68.7 | −47.8 | −33.3 | −23.1 | −15.9 | −10.7 |
| 0.3999 | −1187.4 | −761.4 | −498.8 | −333.0 | −226.0 | −155.6 | −108.4 | −76.3 | −54.1 | −38.6 | −27.6 | −19.7 | −14.0 |
| 0.5000 | −1104.9 | −713.7 | −471.6 | −317.9 | −218.2 | −152.1 | −107.6 | −77.0 | −55.7 | −40.6 | −29.8 | −22.0 | −16.3 |
| 0.6004 | −951.0 | −618.8 | −411.8 | −279.6 | −193.3 | −135.8 | −96.7 | −69.7 | −50.8 | −37.3 | −27.6 | −20.5 | −15.3 |
| 0.7003 | −752.0 | −490.0 | −326.6 | −222.2 | −153.8 | −108.2 | −77.1 | −55.6 | −40.5 | −29.7 | −21.9 | −16.2 | −12.0 |
| 0.8002 | −518.7 | −338.1 | −225.6 | −153.6 | −106.5 | −75.1 | −53.7 | −38.9 | −28.4 | −21.0 | −15.6 | −11.6 | −8.7 |
| 0.8999 | −259.5 | −169.4 | −113.1 | −77.1 | −53.5 | −37.7 | −27.0 | −19.5 | −14.2 | −10.5 | −7.8 | −5.8 | −4.3 |
| 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| η | κ | |||||||
|---|---|---|---|---|---|---|---|---|
| x1 | ηo (mPa·s) | To (K) | B (K) | R2a | κo (mS·cm−1) | To (K) | B (K) | R2a |
| 0 | 0.24 | 190.3 | 967.8 | 0.999 | 24.9 | 230.7 | 365.5 | 0.999 |
| 0.1015 | 0.282 | 193.4 | 888 | 0.999 | 52.2 | 206.9 | 540.4 | 0.999 |
| 0.2000 | 0.295 | 194.4 | 836.9 | 0.999 | 43.6 | 206.6 | 493.1 | 0.999 |
| 0.3007 | 0.292 | 193.8 | 800.9 | 0.999 | 53.3 | 198.5 | 533.3 | 0.999 |
| 0.3999 | 0.277 | 191.2 | 780.7 | 0.999 | 16.2 | 223.7 | 281.9 | 0.999 |
| 0.5000 | 0.263 | 187.4 | 764.5 | 0.999 | 11.3 | 231.3 | 222.1 | 0.999 |
| 0.6004 | 0.204 | 179.9 | 795.2 | 0.999 | 10.0 | 229.1 | 212.5 | 0.999 |
| 0.7003 | 0.129 | 172.7 | 856.9 | 0.999 | 8.7 | 227.5 | 192.8 | 0.999 |
| 0.8002 | 0.102 | 176.2 | 831 | 0.999 | 6.6 | 227.2 | 178.9 | 0.999 |
| 0.8999 | 0.123 | 186 | 718.2 | 0.999 | 3.4 | 235.1 | 144.6 | 0.999 |
| 1 | 0.471 | 198.5 | 455.6 | 0.999 | 25.6 | 202.3 | 818.1 | 0.999 |
| x1 | n | 103 Δxn | 103 ΔΨn | ΔxR | ΔΨR |
|---|---|---|---|---|---|
| 0 | 1.4586 | 0 | 0 | 0 | 0 |
| 0.1015 | 1.4599 | 1.310 | 1.308 | −0.3224 | −0.6806 |
| 0.2000 | 1.4604 | 1.820 | 1.816 | −0.6385 | −1.2827 |
| 0.3007 | 1.4609 | 2.330 | 2.325 | −0.7403 | −1.6094 |
| 0.3999 | 1.4611 | 2.540 | 2.533 | −0.6990 | −1.7176 |
| 0.5000 | 1.4612 | 2.650 | 2.643 | −0.4559 | −1.5466 |
| 0.6004 | 1.4611 | 2.560 | 2.553 | −0.3091 | −1.3859 |
| 0.7003 | 1.4608 | 2.270 | 2.264 | −0.2514 | −1.2212 |
| 0.8002 | 1.4605 | 1.980 | 1.975 | −0.2036 | −0.9649 |
| 0.8999 | 1.4599 | 1.390 | 1.387 | −0.1746 | −0.6169 |
| 1 | 1.4585 | 0 | 0 | 0 | 0 |
| T/K | A0 | A1 | A2 | A3 | A4 | σ |
|---|---|---|---|---|---|---|
| VE/cm3 mol−1 | ||||||
| 293.15 | −11.107 | −14.337 | −10.953 | 15.942 | 6.0212 | 0.087333 |
| 298.15 | −11.271 | −14.371 | −11.168 | 16.012 | 6.4747 | 0.082121 |
| 303.15 | −11.425 | −14.401 | −11.355 | 16.083 | 6.8586 | 0.078529 |
| 308.15 | −11.569 | −14.429 | −11.511 | 16.156 | 7.1722 | 0.076233 |
| 313.15 | −11.704 | −14.455 | −11.638 | 16.229 | 7.4151 | 0.074909 |
| 318.15 | −11.83 | −14.477 | −11.734 | 16.304 | 7.5866 | 0.074295 |
| 323.15 | −11.946 | −14.285 | −11.801 | 16.379 | 7.6863 | 0.077588 |
| 328.15 | −12.052 | −14.514 | −11.836 | 16.456 | 7.7134 | 0.074634 |
| 333.15 | −12.149 | −14.529 | −11.841 | 16.535 | 7.6676 | 0.075618 |
| 338.15 | −12.236 | −14.54 | −11.815 | 16.614 | 7.548 | 0.077371 |
| 343.15 | −12.313 | −14.549 | −11.757 | 16.695 | 7.3543 | 0.080197 |
| 348.15 | −12.38 | −14.555 | −11.668 | 16.777 | 7.0857 | 0.084452 |
| 353.15 | −12.436 | −14.558 | −11.547 | 16.861 | 6.7417 | 0.090499 |
| Δη/mPa·s | ||||||
| 293.15 | −4402.7 | −2700.8 | −1378.1 | 1669.7 | 2724.6 | 11.464135 |
| 298.15 | −2852.6 | −1531.9 | −553.24 | 438.22 | 803.55 | 2.679943 |
| 303.15 | −1888.4 | −885.33 | −175.33 | −36 | 41.466 | 0.836423 |
| 308.15 | −1274.4 | −517.5 | −5.2237 | −192.58 | −228.25 | 1.451973 |
| 313.15 | −874.81 | −303.39 | 66.785 | −219.52 | −292.5 | 1.395598 |
| 318.15 | −609.61 | −176.48 | 92.393 | −197.67 | −276.01 | 1.101902 |
| 323.15 | −430.41 | −100.24 | 96.438 | −161.37 | −232.64 | 0.794003 |
| 328.15 | −308.33 | −64.397 | 117.64 | −82.435 | −258.8 | 1.295174 |
| 333.15 | −221.49 | −26.014 | 81.89 | −92.312 | −141.62 | 0.448191 |
| 338.15 | −160.83 | −9.0716 | 71.941 | −65.834 | −105.27 | 0.431571 |
| 343.15 | −117.42 | 1.0083 | 62.409 | −44.859 | −76.01 | 0.461003 |
| 348.15 | −86.004 | 6.8152 | 53.81 | −28.597 | −53.013 | 0.496660 |
| 353.15 | −63.043 | 9.9539 | 46.291 | −16.169 | −35.213 | 0.523728 |
| Δxn | ||||||
| 293.15 | 1.06 × 10−2 | −0.06 × 10−3 | −0.4 × 10−3 | −1 × 10−3 | 11 × 10−3 | 0.485 × 10−4 |
| 298.15 | 1.22 × 10−2 | −1.3 × 10−3 | 3.3 × 10−3 | 0.8 × 10−3 | 6.9 × 10−3 | 0.304 × 10−4 |
| 303.15 | 1.3 × 10−2 | −1.7 × 10−3 | 6.8 × 10−3 | 2.4 × 10−3 | 5.1 × 10−3 | 0.688 × 10−4 |
| 308.15 | 1.48 × 10−2 | −1.5 × 10−3 | 4.7 × 10−3 | 1.8 × 10−3 | 9.1 × 10−3 | 0.845 × 10−4 |
| ΔΨn against x1 | ||||||
| 293.15 | 1.06 × 10−2 | −0.055 × 10−3 | −0.4 × 10−3 | −1 × 10−3 | 11 × 10−3 | 0.469 × 10−4 |
| 298.15 | 1.17 × 10−2 | −1.2 × 10−3 | 3.3 × 10−3 | 0.8 × 10−3 | 6.9 × 10−3 | 0.312 × 10−4 |
| 303.15 | 1.26 × 10−2 | −1.6 × 10−3 | 6.7 × 10−3 | 2.4 × 10−3 | 5.1 × 10−3 | 0.686 × 10−4 |
| 308.15 | 1.41 × 10−2 | −1.4 × 10−3 | 4.7 × 10−3 | 1.8 × 10−3 | 9.1 × 10−3 | 0.847 × 10−4 |
| ΔΨn against Ψ1 | ||||||
| 293.15 | 1.05 × 10−2 | 1.8 × 10−3 | −0.2 × 10−3 | 0.2 × 10−3 | 11.5 × 10−3 | 0.73 × 10−4 |
| 298.15 | 1.17 × 10−2 | 0.5 × 10−3 | 1.9 × 10−3 | 3.3 × 10−3 | 9.2 × 10−3 | 0.49 × 10−4 |
| 303.15 | 1.27 × 10−2 | −0.4 × 10−3 | 4.2 × 10−3 | 6.4 × 10−3 | 8.7 × 10−3 | 0.85 × 10−4 |
| 308.15 | 1.42 × 10−2 | 0.4 × 10−3 | 2.9 × 10−3 | 5.3 × 10−3 | 12 × 10−3 | 1.19 × 10−4 |
| ΔxR | ||||||
| 293.15 | −1.9293 | −3.8038 | −3.0402 | 4.3952 | 2.8158 | 0.025 |
| 298.15 | −1.8058 | −3.8961 | −2.6609 | 4.5773 | 2.4094 | 0.0255 |
| 303.15 | −1.7031 | −3.9151 | −2.306 | 4.7678 | 2.2485 | 0.0281 |
| 308.15 | −1.5286 | −3.8653 | −2.5239 | 4.7144 | 2.6929 | 0.025 |
| ΔΨR against x1 | ||||||
| 293.15 | −6.2921 | −3.1958 | −3.1249 | 4.4072 | 2.8141 | 0.025 |
| 298.15 | −6.192 | −3.2867 | −2.7455 | 4.5892 | 2.4077 | 0.0254 |
| 303.15 | −6.0536 | −3.3125 | −2.3895 | 4.7795 | 2.2468 | 0.0281 |
| 308.15 | −5.8814 | −3.2642 | −2.6069 | 4.7261 | 2.6912 | 0.025 |
| ΔΨR against Ψ1 | ||||||
| 293.15 | −5.7634 | −3.9989 | −6.3897 | 2.2381 | 5.7545 | 0.0279 |
| 298.15 | −5.6501 | −4.1099 | −6.1522 | 2.5598 | 5.5137 | 0.0271 |
| 303.15 | −5.5082 | −4.1715 | −5.9053 | 2.9193 | 5.5117 | 0.0306 |
| 308.15 | −5.3499 | −4.0698 | −6.0323 | 2.8287 | 5.8762 | 0.031 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, T.-Y.; Chen, B.-K.; Hao, L.; Lin, Y.-C.; Wang, H.P.; Kuo, C.-W.; Sun, I.-W. Physicochemical Properties of Glycine-Based Ionic Liquid [QuatGly-OEt][EtOSO3] (2-Ethoxy-1-ethyl-1,1-dimethyl-2-oxoethanaminium ethyl sulfate) and Its Binary Mixtures with Poly(ethylene glycol) (Mw = 200) at Various Temperatures. Int. J. Mol. Sci. 2011, 12, 8750-8772. https://doi.org/10.3390/ijms12128750
Wu T-Y, Chen B-K, Hao L, Lin Y-C, Wang HP, Kuo C-W, Sun I-W. Physicochemical Properties of Glycine-Based Ionic Liquid [QuatGly-OEt][EtOSO3] (2-Ethoxy-1-ethyl-1,1-dimethyl-2-oxoethanaminium ethyl sulfate) and Its Binary Mixtures with Poly(ethylene glycol) (Mw = 200) at Various Temperatures. International Journal of Molecular Sciences. 2011; 12(12):8750-8772. https://doi.org/10.3390/ijms12128750
Chicago/Turabian StyleWu, Tzi-Yi, Bor-Kuan Chen, Lin Hao, Yuan-Chung Lin, H. Paul Wang, Chung-Wen Kuo, and I-Wen Sun. 2011. "Physicochemical Properties of Glycine-Based Ionic Liquid [QuatGly-OEt][EtOSO3] (2-Ethoxy-1-ethyl-1,1-dimethyl-2-oxoethanaminium ethyl sulfate) and Its Binary Mixtures with Poly(ethylene glycol) (Mw = 200) at Various Temperatures" International Journal of Molecular Sciences 12, no. 12: 8750-8772. https://doi.org/10.3390/ijms12128750




