Swelling-Activated Anion Channels Are Essential for Volume Regulation of Mouse Thymocytes
Abstract
:1. Introduction
2. Results and Discussion
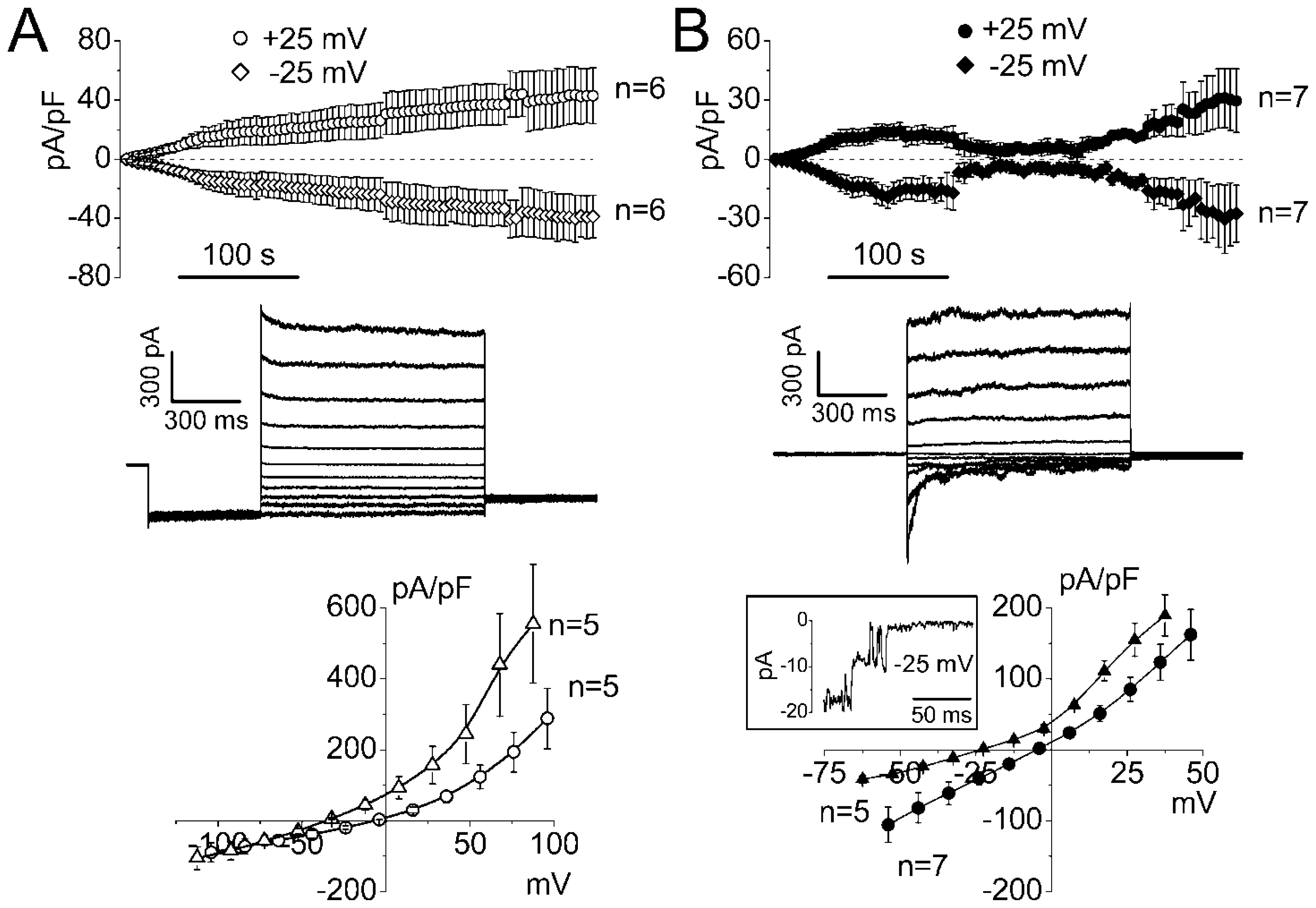
2.1. Whole-Cell Anion Currents Activated in Mouse Thymocytes in Response to Osmotic Cell Swelling
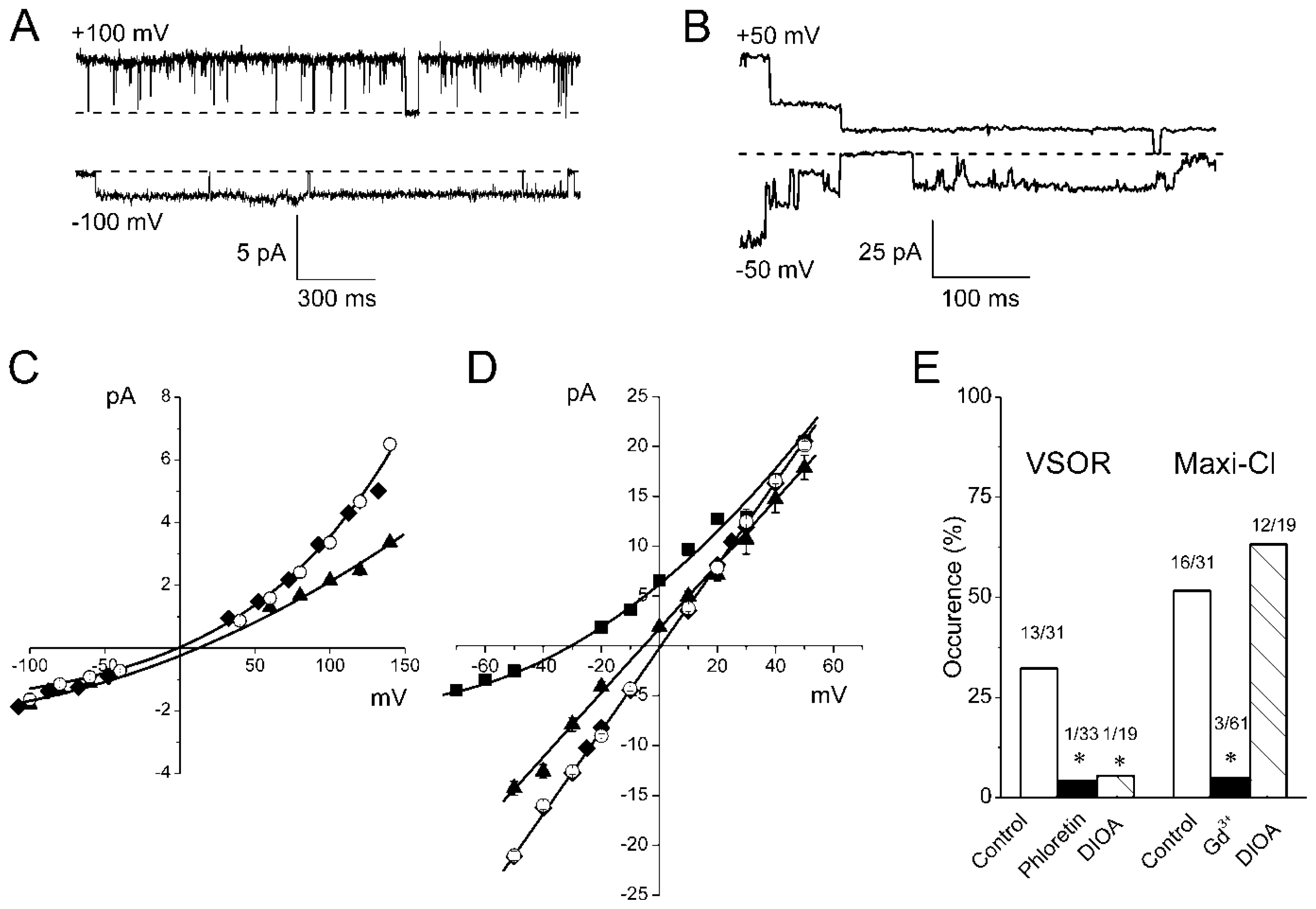
2.2. Single Anion Channel Currents Activated in Osmotically Swollen Mouse Thymocytes
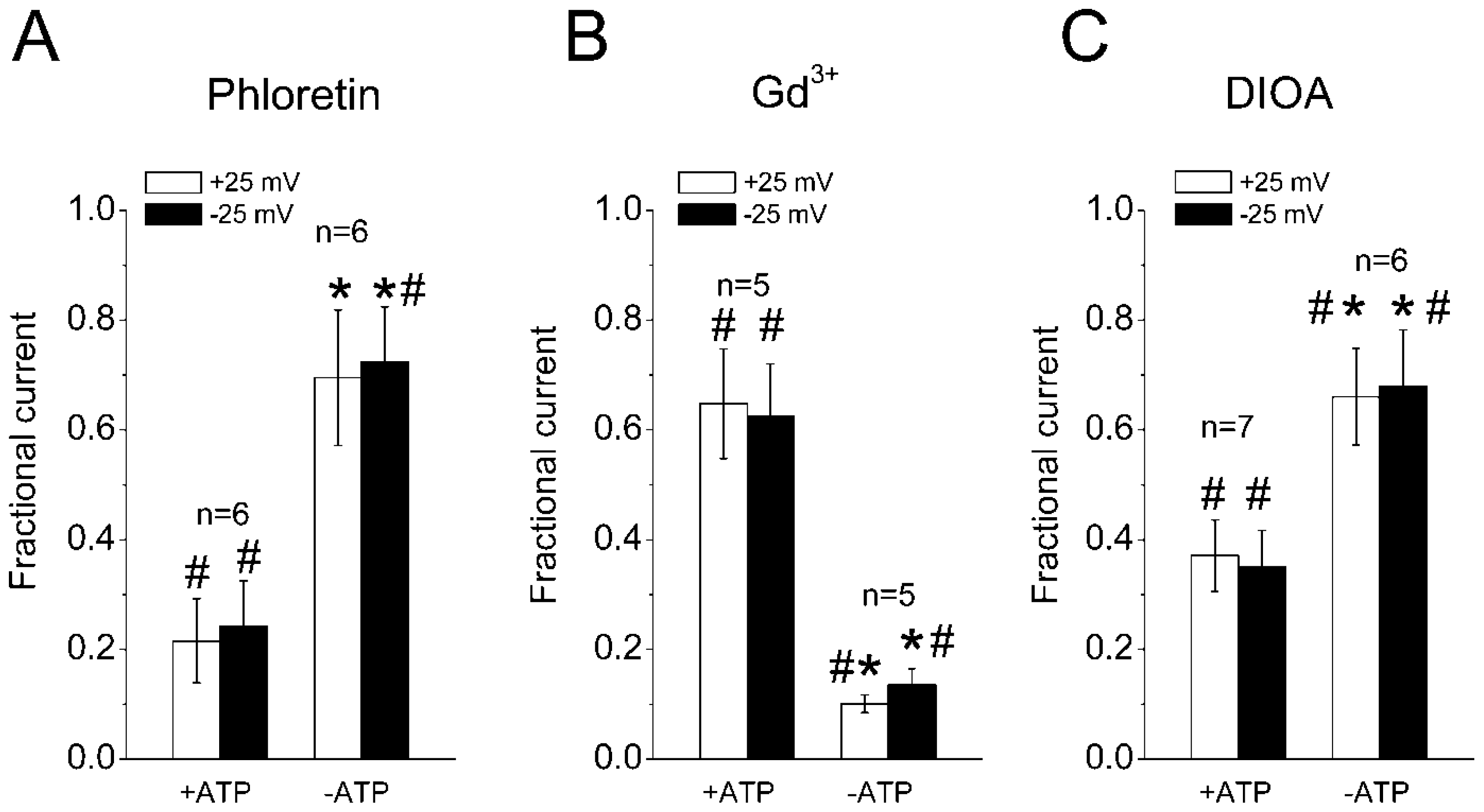
2.3. Sensitivity of the Regulatory Volume Decrease to Blockers for VSOR Cl− Channel and K-Cl Cotransport
3. Experimental Section
3.1. Solutions
3.2. Cells
3.3. Cell Volume Measurements
3.4. Electrophysiology
3.5. Data Analysis
4. Conclusions
Acknowledgments
References
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev 2009, 89, 193–277. [Google Scholar]
- Okada, Y. Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem. Biophys 2004, 41, 233–258. [Google Scholar]
- Wehner, F.; Olsen, H.; Tinel, H.; Kinne-Saffran, E.; Kinne, R.K. Cell volume regulation: Osmolytes, osmolyte transport, and signal transduction. Rev. Physiol. Biochem. Pharmacol 2003, 148, 1–80. [Google Scholar]
- Okada, Y.; Maeno, E.; Shimizu, T.; Dezaki, K.; Wang, J.; Morishima, S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol 2001, 532, 3–16. [Google Scholar]
- Okada, Y.; Maeno, E. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp. Biochem. Physiol. A Mol. Integr. Physiol 2001, 130, 377–383. [Google Scholar]
- Okada, Y.; Maeno, E.; Shimizu, T.; Manabe, K.; Mori, S.; Nabekura, T. Dual roles of plasmalemmal chloride channels in induction of cell death. Pflugers Arch 2004, 448, 287–295. [Google Scholar]
- Okada, Y.; Shimizu, T.; Maeno, E.; Tanabe, S.; Wang, X.; Takahashi, N. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J. Membr. Biol 2006, 209, 21–29. [Google Scholar]
- Okada, Y.; Sato, K.; Toychiev, A.H.; Suzuki, M.; Dutta, A.K.; Inoue, H.; Sabirov, R. The Puzzles of Volume-Activated Anion Channels. In Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: from Molecules to Diseases; Alvarez-Leefmans, F.J., Delpire, E., Eds.; Elsevier: San Diego, CA, USA, 2009. [Google Scholar]
- Okada, Y.; Sato, K.; Numata, T. Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J. Physiol 2009, 587, 2141–2149. [Google Scholar]
- Hernandez, J.B.; Newton, R.H.; Walsh, C.M. Life and death in the thymus—Cell death signaling during T cell development. Curr. Opin. Cell Biol 2010, 22, 865–871. [Google Scholar]
- Wiegers, G.J.; Kaufmann, M.; Tischner, D.; Villunger, A. Shaping the T-cell repertoire: A matter of life and death. Immunol. Cell Biol 2011, 89, 33–39. [Google Scholar]
- Arrazola, A.; Rota, R.; Hannaert, P.; Soler, A.; Garay, R.P. Cell volume regulation in rat thymocytes. J. Physiol 1993, 465, 403–414. [Google Scholar]
- Kurbannazarova, R.S.; Tashmukhamedov, B.A.; Sabirov, R.Z. Osmotic water permeability and regulatory volume decrease of rat thymocytes. Gen. Physiol. Biophys 2003, 22, 221–232. [Google Scholar]
- Kurbannazarova, R.S.; Tashmukhamedov, B.A.; Sabirov, R.Z. Role of potassium and chlorine channels in the regulation of thymocyte volume in rats. Bull. Exp. Biol. Med 2008, 145, 606–609. [Google Scholar]
- Soler, A.; Rota, R.; Hannaert, P.; Cragoe, E.J., Jr; Garay, R.P. Volume-dependent K+ and Cl− fluxes in rat thymocytes. J. Physiol. 1993, 465, 387–401. [Google Scholar]
- Kubo, M.; Okada, Y. Volume-regulatory Cl− channel currents in cultured human epithelial cells. J. Physiol 1992, 456, 351–371. [Google Scholar]
- Okada, Y. Volume expansion-sensing outward-rectifier Cl− channel: Fresh start to the molecular identity and volume sensor. Am. J. Physiol 1997, 273, C755–C789. [Google Scholar]
- Sabirov, R.Z.; Okada, Y. The maxi-anion channel: A classical channel playing novel roles through an unidentified molecular entity. J. Physiol. Sci 2009, 59, 3–21. [Google Scholar]
- Dutta, A.K.; Sabirov, R.Z.; Uramoto, H.; Okada, Y. Role of ATP-conductive anion channel in ATP release from neonatal rat cardiomyocytes in ischaemic or hypoxic conditions. J. Physiol 2004, 559, 799–812. [Google Scholar]
- Liu, H.T.; Tashmukhamedov, B.A.; Inoue, H.; Okada, Y.; Sabirov, R.Z. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia 2006, 54, 343–357. [Google Scholar]
- Liu, H.T.; Sabirov, R.Z.; Okada, Y. Oxygen-glucose deprivation induces ATP release via maxi-anion channels in astrocytes. Purinergic Signal 2008, 4, 147–154. [Google Scholar]
- Sabirov, R.Z.; Dutta, A.K.; Okada, Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J. Gen. Physiol 2001, 118, 251–266. [Google Scholar]
- Fan, H.T.; Kida, H.; Morishima, S.; Oiki, S.; Okada, Y. Effects of phloretin on chloride channels in epithelilal cells. Jpn. J. Physiol 1999, 49, S109. [Google Scholar]
- Botchkin, L.M.; Matthews, G. Swelling activates chloride current and increases internal calcium in nonpigmented epithelial cells from the rabbit ciliary body. J. Cell. Physiol 1995, 164, 286–294. [Google Scholar]
- Okada, Y.; Petersen, C.C.; Kubo, M.; Morishima, S.; Tominaga, M. Osmotic swelling activates intermediate-conductance Cl− channels in human intestinal epithelial cells. Jpn. J. Physiol 1994, 44, 403–409. [Google Scholar]
- Ternovsky, V.I.; Okada, Y.; Sabirov, R.Z. Sizing the pore of the volume-sensitive anion channel by differential polymer partitioning. FEBS Lett 2004, 576, 433–436. [Google Scholar]
- Tsumura, T.; Oiki, S.; Ueda, S.; Okuma, M.; Okada, Y. Sensitivity of volume-sensitive Cl− conductance in human epithelial cells to extracellular nucleotides. Am. J. Physiol 1996, 271, C1872–C1878. [Google Scholar]
- Abdullaev, I.F.; Sabirov, R.Z.; Okada, Y. Upregulation of swelling-activated Cl− channel sensitivity to cell volume by activation of EGF receptors in murine mammary cells. J. Physiol 2003, 549, 749–758. [Google Scholar]
- Jackson, P.S.; Strange, K. Single-channel properties of a volume-sensitive anion conductance. current activation occurs by abrupt switching of closed channels to an open state. J. Gen. Physiol 1995, 105, 643–660. [Google Scholar]
- Nilius, B.; Eggermont, J.; Voets, T.; Buyse, G.; Manolopoulos, V.; Droogmans, G. Properties of volume-regulated anion channels in mammalian cells. Prog. Biophys. Mol. Biol 1997, 68, 69–119. [Google Scholar]
- Sabirov, R.Z.; Prenen, J.; Droogmans, G.; Nilius, B. Extra- and intracellular proton-binding sites of volume-regulated anion channels. J. Membr. Biol 2000, 177, 13–22. [Google Scholar]
- Strange, K.; Emma, F.; Jackson, P.S. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol 1996, 270, C711–C730. [Google Scholar]
- Dutta, A.K.; Okada, Y.; Sabirov, R.Z. Regulation of an ATP-conductive large-conductance anion channel and swelling-induced ATP release by arachidonic acid. J. Physiol 2002, 542, 803–816. [Google Scholar]
- Dutta, A.K.; Korchev, Y.E.; Shevchuk, A.I.; Hayashi, S.; Okada, Y.; Sabirov, R.Z. Spatial distribution of maxi-anion channel on cardiomyocytes detected by smart-patch technique. Biophys. J 2008, 94, 1646–1655. [Google Scholar]
- Liu, H.T.; Toychiev, A.H.; Takahashi, N.; Sabirov, R.Z.; Okada, Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res 2008, 18, 558–565. [Google Scholar]
- Toychiev, A.H.; Sabirov, R.Z.; Takahashi, N.; Ando-Akatsuka, Y.; Liu, H.; Shintani, T.; Noda, M.; Okada, Y. Activation of maxi-anion channel by protein tyrosine dephosphorylation. Am. J. Physiol. Cell Physiol 2009, 297, C990–C1000. [Google Scholar]
- Liu, Y.; Oiki, S.; Tsumura, T.; Shimizu, T.; Okada, Y. Glibenclamide blocks volume-sensitive Cl− channels by dual mechanisms. Am. J. Physiol 1998, 275, C343–C351. [Google Scholar]
- Sabirov, R.Z.; Manjosova, M.A.; Tadjibaeva, E.T.; Krasilnikov, O.V. The interaction of amphotericin B with cell membrane of rat thymocytes. Gen. Physiol. Biophys 1993, 12, 249–257. [Google Scholar]
- Hazama, A.; Okada, Y. Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. J. Physiol 1988, 402, 687–702. [Google Scholar]
- Sabirov, R.Z.; Okada, Y. Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway. Biophys. J 2004, 87, 1672–1685. [Google Scholar]
- Lang, F.; Busch, G.L.; Ritter, M.; Volkl, H.; Waldegger, S.; Gulbins, E.; Haussinger, D. Functional significance of cell volume regulatory mechanisms. Physiol. Rev 1998, 78, 247–306. [Google Scholar]
- Feray, J.C.; Guerrouache, K.; Garay, R.P. Dramatic magnesium efflux induced by high potassium in rat thymocytes. Biochem. Biophys. Res. Commun 2000, 268, 673–676. [Google Scholar]
- Savino, W.; Dardenne, M. Neuroendocrine control of thymus physiology. Endocr. Rev 2000, 21, 412–443. [Google Scholar]
- Sabirov, R.Z.; Okada, Y. ATP-conducting maxi-anion channel: A new player in stress-sensory transduction. Jpn. J. Physiol 2004, 54, 7–14. [Google Scholar]
- Sabirov, R.Z.; Okada, Y. ATP release via anion channels. Purinergic Signal 2005, 1, 311–328. [Google Scholar]

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kurbannazarova, R.S.; Bessonova, S.V.; Okada, Y.; Sabirov, R.Z. Swelling-Activated Anion Channels Are Essential for Volume Regulation of Mouse Thymocytes. Int. J. Mol. Sci. 2011, 12, 9125-9137. https://doi.org/10.3390/ijms12129125
Kurbannazarova RS, Bessonova SV, Okada Y, Sabirov RZ. Swelling-Activated Anion Channels Are Essential for Volume Regulation of Mouse Thymocytes. International Journal of Molecular Sciences. 2011; 12(12):9125-9137. https://doi.org/10.3390/ijms12129125
Chicago/Turabian StyleKurbannazarova, Ranokhon S., Svetlana V. Bessonova, Yasunobu Okada, and Ravshan Z. Sabirov. 2011. "Swelling-Activated Anion Channels Are Essential for Volume Regulation of Mouse Thymocytes" International Journal of Molecular Sciences 12, no. 12: 9125-9137. https://doi.org/10.3390/ijms12129125



