Molecular Recognition of Arginine by Supramolecular Complexation with Calixarene Crown Ether Based on Surface Plasmon Resonance
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Formation and Characterization of Calixcrown Ether SAM
2.3. Recognition of Arginine on the Calix[4]Crown Monolayer
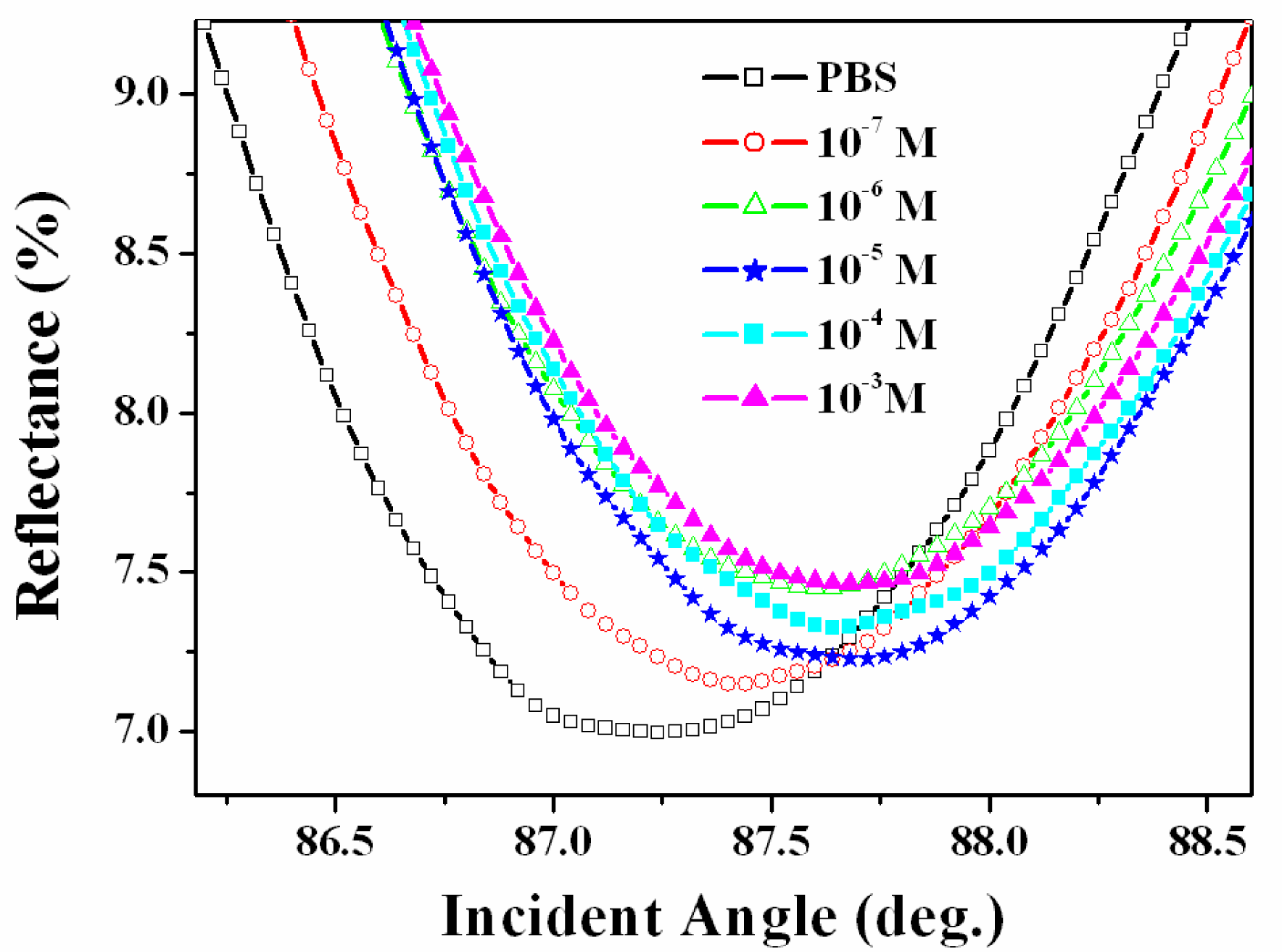
3. Results and Discussion
3.1. Characterization of Calix[4]Crown SAM
3.2. Recognition of Arginine on the Calix[4]Crown Monolayer
4. Conclusions
Acknowledgments
References
- Tapiero, H; Mathé, G; Couvreur, P; Tew, KD. l-Arginine. Biomed. Pharmacother 2002, 56, 439–445. [Google Scholar]
- Stechmiler, JK; Childress, B; Cowan, L. Arginine supplementation and wound healing. Nutr. Clin. Pract 2005, 20, 52–61. [Google Scholar]
- Witte, MB; Barbul, A. Arginine Physiology and its implication for wound healing. Wound Repair Regen 2003, 11, 419–423. [Google Scholar]
- Stanislavov, R; Nikolova, V. Treatment of erectile dysfunction with pycnogenol and l-arginine. J. Sex Marital Ther 2003, 29, 207–213. [Google Scholar]
- Lebret, T; Hervéa, JM; Gornyb, P; Worcelc, M; Botto, H. Efficacy and safety of a novel combination of l-arginine glutamate and yohimbine hydrochloride. Eur. Urol 2002, 41, 608–613. [Google Scholar]
- Nascimento, MM; Gordan, VV; Garvan, CW; Browngardt, CM; Burne, RA. Correlations of Oral Bacterial Arginine and Urea Catabolism with Caries Experience. Microbiol. Immun 2009, 24, 89–95. [Google Scholar]
- Wimmer, F; Oberwinkler, T; Bisle, B; Tittor, J; Oesterhelt, D. Identification of the arginine/ornithine antiporter ArcD from Halobacterium Salinarum. FEBS Lett 2008, 582, 3771–3775. [Google Scholar]
- Fang, Y; Shane, T; Wu, F; Williams, C; Miller, C. The structure and transport mechanism of AdiC-an arginine/agmatine antiporter. Biophys. J 2010, 98, 418a. [Google Scholar]
- Julian, RR; Beauchamp, JL. Site specific sequestering and stabilization of charge in peptides by supramolecular adduct formation with 18-crown-6 ether by way of electrospray ionization. Int. J. Mass. Spectrom 2001, 210, 613–623. [Google Scholar]
- Julian, RR; Akin, M; May, JA; Stoltz, BM; Beauchamp, JL. Molecular recognition of arginine in small peptides by supramolecular complexation with dibenzo-30-crown-10 ether. Int. J. Mass. Spectrom 2002, 220, 87–96. [Google Scholar]
- Friess, SD; Zenobi, R. Protein structure information from mass spectrometry? Selective titration of arginine residues by sulfonates. J. Am. Soc. Mass Spectrom 2001, 12, 810–818. [Google Scholar]
- Ngola, SM; Kearney, PC; Mecozzi, S; Russell, K; Dougherty, DA. A selective receptor for arginine derivatives in aqueous media. Energetic consequences of salt bridges that are highly exposed to water. J. Am. Chem. Soc 1999, 121, 1192–1201. [Google Scholar]
- Rensing, S; Arendt, A; Springer, A; Grawe, T; Schrader, T. Optimization of a synthetic arginine receptor. Systematic tuning of noncovalent interactions. J. Org. Chem 2001, 66, 5814–5821. [Google Scholar]
- Schrader, TH. Strong binding of arylguanidinium ions by benzylic bisphosphonates—evidence for π-cation and π,π-interactions. Tetrahedron Lett 1998, 39, 517–520. [Google Scholar]
- Bell, TW; Khasanov, AB; Drew, MGB; Filikov, A; James, TL. A small-molecule guanidinium receptor: The arginine cork. Angew. Chem. Int. Ed 1999, 38, 2543–2547. [Google Scholar]
- Stone, MM; Franz, AH; Lebrilla, CB. Non-covalent calixarene amino acid complexes formed by MALDI-MS. J. Am. Soc. Mass Spectr 2002, 13, 964–974. [Google Scholar]
- Koh, K; Araki, K; Shinkai, S; Asfari, Z; Vicens, J. Cation binding properties of a novel 1,3-alternate calix[4]biscrown. formation of 1:1 and 1:2 complexes and unique cation tunneling across a calix[4]arene cavity. Tetrahedron Lett 1995, 36, 6095–6098. [Google Scholar]
- Mutihac, L; Buschmann, HJ; Mutihac, RC; Schollmeyer, E. Complexation and separation of amines, amino acids, and peptides by functionalized calix[n]arenas. J. Incl. Phenom. Macrocyclic Chem 2005, 51, 1–10. [Google Scholar]
- Ikeda, A; Shinkai, S. Novel cavity design using calix[n]arene skeletons: Towards molecular recognition and metal binding. Chem. Rev 1997, 97, 1713–1734. [Google Scholar]
- Chen, H; Gal, YS; Kim, SH; Choi, HJ; Oh, MC; Lee, J; Koh, K. Potassium ion sensing using a self-assembled calix[4]crown monolayer by surface plasmon resonance. Sens. Actuators B 2008, 133, 577–581. [Google Scholar]
- Ludwig, R. Calixarenes for biochemical recognition and separation. Microchim. Acta 2005, 152, 1–19. [Google Scholar]
- Arena, G; Casnati, A; Contino, A; Magri, A; Sansone, F; Sciotto, D; Ungaro, R. Inclusion of naturally occuring amino acids in water soluble calix[4]arenes: A microcalorimetric and 1H NMR investigation supported by molecular modeling. Org. Biomol. Chem 2006, 4, 243–249. [Google Scholar]
- Antipin, IS; Stoikov, II; Pinkhassik, EM; Fitseva, NA; Stibor, I; Konovalov, AI. calix[4]arene based-aminophosphonates: Novel carriers for awitterionic amino acids transport. Tetrahedron Lett 1997, 38, 5865–5868. [Google Scholar]
- Glennon, JD; Horne, E; Hall, K; Cocker, D; Kuhn, A; Harris, SJ; McKervey, MA. Silica-bonded calixarenes in chromatography. II. Chromatographic retention of metal ions and amino acid ester hydrochlorides. J. Chromatogr. A 1996, 731, 47–55. [Google Scholar]
- Lee, Y; Lee, EK; Cho, YW; Matsui, T; Kang, IC; Kim, TS; Han, MH. ProteoChip: A highly sensitive protein microarray prepared by a novel method of protein immobilization for application of protein-protein interaction studies. Proteomics 2003, 3, 2289–2304. [Google Scholar]
- Oh, SW; Moon, JD; Lim, HJ; Park, SY; Kim, T; Park, J; Han, MH; Snyder, M; Choi, EY. Calixarene derivative as a tool for highly sensitive detection and oriented immobilization of proteins in a microarray format through noncovalent molecular interaction. FASEB 2005, 1335–1337. [Google Scholar]
- Bard, J; Faulkner, LR. Electrochemical Methods: Fundamentals and Application; John Wiley & Sons: New York, NY, USA, 2001; pp. 239–243. [Google Scholar]
- Chen, H; Lee, M; Lee, J; An, WG; Choi, HJ; Kim, SH; Koh, K. Building a novel vitronectin Assay by immobilization of integrin on calixarene monolayer. Talanta 2008, 75, 99–103. [Google Scholar]
- Ghidini, E; Ugozzoli, F; Ungaro, R; Harkema, S; El-Fadl, AA; Reinhoudt, DN. Complexation of alkali metal cations by conformationally rigid, stereoisomeric calix[4]arene crown ethers: a auantitative evaluation of preorganization. J. Am. Chem. Soc 1990, 112, 6979–6985. [Google Scholar]
- Gawley, RE; Pinet, S; Cardona, C; Datta, P; Ren, T; Guida, W; Nydick, J; Leblanc, RM. Chemosensors for the marine toxin saxitoxin. J. Am. Chem. Soc 2002, 124, 13448–13453. [Google Scholar]




| [arg.] (M) | 1.0 × 10−7 | 1.0 × 10−6 | 1.0 × 10−5 | 1.0 × 10−4 | 1.0 × 10−3 |
| RI | 1.323 | 1.365 | 1.387 | 1.405 | 1.406 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, H.; Gu, L.; Yin, Y.; Koh, K.; Lee, J. Molecular Recognition of Arginine by Supramolecular Complexation with Calixarene Crown Ether Based on Surface Plasmon Resonance. Int. J. Mol. Sci. 2011, 12, 2315-2324. https://doi.org/10.3390/ijms12042315
Chen H, Gu L, Yin Y, Koh K, Lee J. Molecular Recognition of Arginine by Supramolecular Complexation with Calixarene Crown Ether Based on Surface Plasmon Resonance. International Journal of Molecular Sciences. 2011; 12(4):2315-2324. https://doi.org/10.3390/ijms12042315
Chicago/Turabian StyleChen, Hongxia, Limin Gu, Yongmei Yin, Kwangnak Koh, and Jaebeom Lee. 2011. "Molecular Recognition of Arginine by Supramolecular Complexation with Calixarene Crown Ether Based on Surface Plasmon Resonance" International Journal of Molecular Sciences 12, no. 4: 2315-2324. https://doi.org/10.3390/ijms12042315




