Immobilization of Glucose Oxidase in Alginate-Chitosan Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microsphere Preparation
2.3. Activity Assay for Free and Encapsulated GOX
2.4. Characterization of CACM
2.5. Optimization of Immobilization Conditions
2.5.1. Encapsulation pH
2.5.2. Chitosan Molecular Weight
2.5.3. Encapsulation Time and Chitosan Concentration
2.6. Thermal and Storage Stabilities of Free GOX and CACM-GOX
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of CACM
3.2. Optimization of the Encapsulation Conditions
3.2.1. Effects of pH on GOX Encapsulation
3.2.2. Effect of Chitosan Molecular Weight on GOX Encapsulation
3.2.3. Effects of Encapsulation Time and Chitosan Concentration on GOX Immobilization
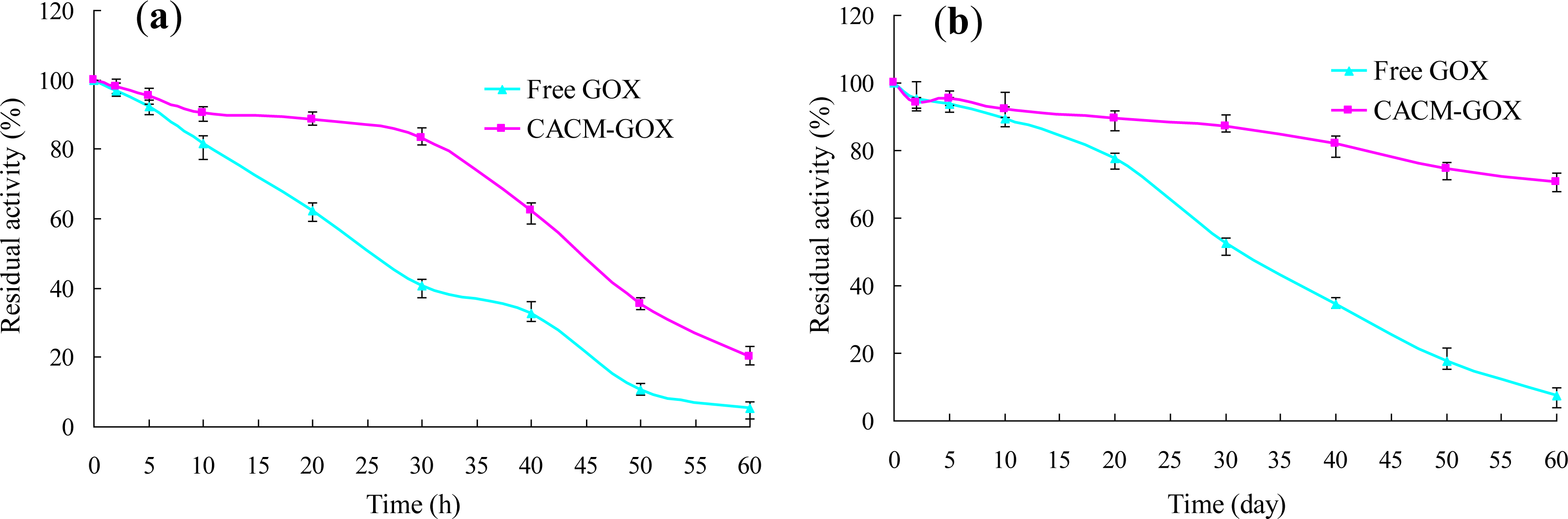
3.3. Thermal and Storage Stabilities of Free GOX and CACM-GOX
4. Conclusions
Acknowledgments
References
- Bonet, A; Rosell, CM; Caballero, PA; Gómez, M; Pérez-Munuera, I; Lluch, MA. Glucose oxidase effect on dough rheology and bread quality: A study from macroscopic to molecular level. Food Chem 2006, 99, 408–415. [Google Scholar]
- Caballero, PA; Gómez, M; Rosell, CM. Bread quality and dough rheology of enzyme-supplemented wheat flour. Eur. Food Res. Technol 2007, 224, 525–534. [Google Scholar]
- Dunnewind, B; van Vliet, T; Orsel, R. Effect of oxidative enzymes on bulk rheological properties properties of wheat flour doughs. J. Cereal Sci 2002, 36, 357–366. [Google Scholar]
- Poulsen, C; Hostrup, PB. Purification and characterization of a hexose oxidase with excellent strengthening effects in bread. Cereal Chem 1998, 75, 51–57. [Google Scholar]
- Rasiah, IA; Sutton, KH; Low, FL; Lin, HM; Gerrard, JA. Crosslinking of wheat dough proteins by glucose oxidase and the resulting effects on bread and croissants. Food Chem 2005, 89, 325–332. [Google Scholar]
- Vemulapalli, V; Miller, KA; Hoseney, RC. Glucose oxidase in breadmaking systems. Cereal Chem 1998, 75, 439–442. [Google Scholar]
- Vemulapalli, V; Hoseney, RC. Glucose oxidase effects on gluten and water solubles. Cereal Chem 1998, 75, 859–862. [Google Scholar]
- Indrani, D; Prabhasankar, P; Rajiv, J; Rao, GV. Scanning electron microscopy, rheological characteristics, and bread-baking performance of wheat-flour dough as affected by enzymes. J. Food Sci 2003, 68, 2804–2809. [Google Scholar]
- Primo-Martín, C; Wang, MW; Lichtendonk, WJ; Plijter, JJ; Hamer, RJ. An explanation for the combined effect of xylanase-glucose oxidase in dough systems. J. Sci. Food Agric 2005, 85, 1186–1196. [Google Scholar]
- Primo-Martin, C; Valera, R; Martinez-Anaya, MA. Effect of pentosanase and oxidases on the characteristics of doughs and the glutenin macropolymer (GMP). J. Agric. Food Chem 2003, 51, 4673–4679. [Google Scholar]
- Rakotozafy, L; Mackova, B; Delcros, J; Boussard, A; Davidou, S; Potus, J; Nicolas, J. Effect of adding exogenous oxidative enzymes on the activity of three endogenous oxidoreductases during mixing of wheat flour dough. Cereal Chem 1999, 76, 213–218. [Google Scholar]
- Li, XY; Jin, LJ; McAllister, TA; Stanford, K; Xu, JY; Lu, YN; Zhen, YH; Sun, YX; Xu, YP. Chitosan-alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY). J. Agric. Food Chem 2007, 55, 2911–2917. [Google Scholar]
- Taqieddin, E; Amiji, M. Enzyme immobilization in novel alginate-chitosan core-shell microcapsules. Biomaterials 2004, 25, 1937–1945. [Google Scholar]
- Poncelet, D; Lencki, R; Beaulieu, C; Halle, JP; Neufeld, RJ; Fournier, A. Production of alginate beads by emulsification/internal gelation: Methodology. Appl. Microbiol. Biot 1992, 38, 39–45. [Google Scholar]
- Poncelet, D; Poncelet De Smet, B; Beaulieu, C; Huguit, ML; Fournier, A; Neufeld, RJ. Production of alginate beads by emulsification/internal gelation II: Physicochemistry. Appl. Microbiol. Biot 1995, 43, 644–650. [Google Scholar]
- Klotzbach, T; Watt, M; Ansari, Y; Minteer, SD. Effects of hydrophobic modification of chitosan and Nafion on transport properties, ion-exchange capacities, and enzyme immobilization. J. Membr. Sci 2006, 282, 276–283. [Google Scholar]
- Liu, Q; Rauth, AM; Wu, XY. Immobilization and bioactivity of glucose oxidase in hydrogel microspheres formulated by an emulsification-internal gelation-adsorption-polyelectrolyte coating method. Int. J. Pharm 2007, 339, 148–156. [Google Scholar]
- Wang, K; He, Z. Alginate-konjac glucomannan-chitosan beads as controlled release matrix. Int. J. Pharm 2002, 244, 117–126. [Google Scholar]
- Anal, AK; Stevens, WF. Chitosan-alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm 2005, 290, 45–54. [Google Scholar]
- Lin, YH; Liang, HF; Chung, CK; Chen, MC; Sung, HW. Physically crosslinked alginate/N,O-carboxymethyl chitosan hydrogels with calcium for oral delivery of protein drugs. Biomaterials 2005, 26, 2105–2113. [Google Scholar]
- Ribeiro, AJ; Siva, C; Ferreira, D; Veiga, F. Chitosan-reinforced alginate microspheres obtained through the emulsification/internal gelation technique. Eur. J. Pharm. Sci 2005, 25, 31–40. [Google Scholar]
- Miller, GL. Use of dinitro salicylic acid reagent for determination of reducing sugar. Anal. Chem 1959, 31, 426–428. [Google Scholar]
- Sankalia, MG; Mashru, RC; Sankalia, JM; Sutariya, VB. Reversed chitosan-alginate polyelectrolyte complex for stability improvement of alpha-amylase: Optimization and physicochemical characterization. Eur. J. Pharm. Biopharm 2007, 65, 215–232. [Google Scholar]
- Khalid, MN; Agnely, F; Yagoui, N; Grossiord, JL; Couarraze, G. Water sate characterization, swelling behavior, thermal and mechanical properties of chitosan based networks. Eur. J. Pharm. Sci 2000, 15, 425–432. [Google Scholar]
- Ozyilmaz, G; Tukel, SS; Alptekin, O. Activity and storage stability of immobilized glucose oxidase onto magnesium silicate. J. Mol. Catal. B Enzym 2005, 35, 154–160. [Google Scholar]
- Lee, JS; Cha, DS; Park, HJ. Survival of freeze-dried Lactobacillus bulgaricus KFRI 673 in chitosan-coated calcium alginate microparticles. J. Agric. Food Chem 2004, 52, 7300–7305. [Google Scholar]
- Sikorski, P; Mo, F; Skjåk-Bræk, G; Stokke, BT. Evidence for Egg-Box-Compatible Interactions in Calcium-Alginate Gels from Fiber X-ray Diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar]
- Onda, M; Ariga, K; Kunitake, AT. Activity and Stability of Glucose Oxidase in Molecular Films Assembled Alternately with Polyions. J. Biosci. Bioeng 1999, 87, 69–75. [Google Scholar]







| Chitosan Concentration (%, w/w) | The Encapsulation Efficiency of GOX (%) a | The Amount of GOX Loaded (mg/g) a | The Total Activity of CACM-GOX (U) a,b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 2.0 | 0.5 | 1.0 | 2.0 | 0.5 | 1.0 | 2.0 | ||
| Encapsulation time (min) | 10 | 46.7 ± 2.7 | 56.3 ± 3.5 | 55.5 ± 1.9 | 25.1 ± 0.6 | 26.9 ± 0.3 | 25.0 ± 0.2 | 3.51 ± 0.21 | 4.19 ± 0.12 | 3.40 ± 1.21 |
| 20 | 55.3 ± 2.2 | 70.3 ± 2.4 | 70.1 ± 1.1 | 31.5 ± 0.4 | 32.0 ± 0.7 | 31.3 ± 0.3 | 4.20 ± 0.27 | 5.11 ± 0.31 | 4.76 ± 1.19 | |
| 40 | 64.2 ± 1.9 | 83.1 ± 3.1 | 84.5 ± 0.7 | 35.2 ± 0.5 | 37.7 ± 0.5 | 32.2 ± 0.5 | 4.76 ± 0.28 | 6.05 ± 0.23 | 4.82 ± 1.09 | |
| 60 | 76.1 ± 3.2 | 91.1 ± 1.2 | 90.6 ± 2.0 | 37.2 ±0.3 | 41.5 ± 0.4 | 31.4 ± 0.3 | 5.32 ± 0.12 | 6.56 ± 0.24 | 5.72 ± 0.87 | |
| 80 | 75.2 ± 2.4 | 92.5 ± 2.7 | 93.6 ± 1.9 | 37.4 ± 0.7 | 41.6 ± 0.5 | 31.5 ± 0.4 | 5.35 ± 0.22 | 6.35 ± 0.16 | 5.93 ± 1.07 | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, X.; Zhu, K.-X.; Zhou, H.-M. Immobilization of Glucose Oxidase in Alginate-Chitosan Microcapsules. Int. J. Mol. Sci. 2011, 12, 3042-3054. https://doi.org/10.3390/ijms12053042
Wang X, Zhu K-X, Zhou H-M. Immobilization of Glucose Oxidase in Alginate-Chitosan Microcapsules. International Journal of Molecular Sciences. 2011; 12(5):3042-3054. https://doi.org/10.3390/ijms12053042
Chicago/Turabian StyleWang, Xia, Ke-Xue Zhu, and Hui-Ming Zhou. 2011. "Immobilization of Glucose Oxidase in Alginate-Chitosan Microcapsules" International Journal of Molecular Sciences 12, no. 5: 3042-3054. https://doi.org/10.3390/ijms12053042




