Enhancing Effect of Glycerol on the Tensile Properties of Bombyx mori Cocoon Sericin Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Secondary Structure Transition
2.2. Thermal Characterization
2.3. Microscopic Structure Analysis
2.4. Tensile Properties
3. Experimental Section
3.1. Materials
3.2. Sericin Solution Extraction
3.3. Preparation of Sericin Films with and without Glycerol
3.4. Tensile Properties
3.5. Attenuated Total Reflection-Fourier Transform Infrared Analysis (ATR-FTIR)
3.6. Thermogravimetric Analysis (TGA)
3.7. Differential Scanning Calorimetry (DSC)
3.8. Scanning Electron Microscopy (SEM)
4. Conclusions
Acknowledgments
References
- Dang, JM; Leong, KW. Natural polymers for gene delivery and tissue engineering. Adv. Drug Deliv. Rev 2006, 58, 487–499. [Google Scholar]
- Silva, SS; Mano, JF; Reis, RL. Potential applications of natural origin polymer-based systems in soft tissue regeneration. Crit. Rev. Biotechnol 2010, 30, 200–221. [Google Scholar] [Green Version]
- Venkatesan, J; Kim, SK. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar]
- Murphy, AR; Kaplan, DL. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem 2009, 19, 6443–6450. [Google Scholar]
- Levin, B; Rajkhowa, R; Redmond, SL; Atlas, MD. Grafts in myringoplasty: Utilizing a silk fibroin scaffold as a novel device. Expert Rev. Med. Devices 2009, 6, 653–664. [Google Scholar]
- Yang, B; Yin, ZH; Cao, JL; Shi, ZL; Zhang, ZT; Song, HX; Liu, FQ; Caterson, B. In vitro cartilage tissue engineering using cancellous bone matrix gelatin as a biodegradable scaffold. Biomed. Mater 2010, 5, 045003. [Google Scholar]
- Aramwit, P; Sangcakul, A. The effects of sericin cream on wound healing in rats. Biosci. Biotechnol. Biochem 2007, 71, 2473–2477. [Google Scholar]
- Aramwit, P; Kanokpanont, S; Punyarit, P; Srichana, T. Effectiveness of inflammatory cytokines induced by sericin compared to sericin in combination with silver sulfadiazine cream on wound healing. Wounds-Compend. Clin. Res. Pract 2009, 21, 198–206. [Google Scholar]
- Takeuchi, A; Ohtsuki, C; Miyazaki, T; Kamitakahara, M; Ogata, S-I; Yamazaki, M; Furutani, Y; Kinoshita, H; Tanihara, M. Heterogeneous nucleation of hydroxyapatite on protein structural effect of silk sericins. J. R. Soc. Interface 2005, 2, 373–378. [Google Scholar]
- Cai, YR; Jin, J; Mei, DP; Xia, NX; Yao, JM. Effect of silk sericin on assembly of hydroxyapatite nanocrystals into enamel prism-like structure. J. Mater. Chem 2009, 19, 5751–5758. [Google Scholar]
- Cai, Y; Mei, D; Jiang, T; Yao, J. Synthesis of oriented hydroxyapatite crystals: Effect of reaction conditions in the presence or absence of silk sericin. Mater. Lett 2010, 64, 2676–2678. [Google Scholar]
- Zhang, YQ; Ma, Y; Xia, YY; Shen, WD; Mao, JP; Xue, RY. Silk sericin-insulin bioconjugates: Synthesis, characterization and biological activity. J. Control. Release 2006, 115, 307–315. [Google Scholar]
- Oh, H; Lee, JY; Kim, A; Ki, CS; Kim, JW; Park, YH; Lee, KH. Preparation of silk sericin beads using LiCl/DMSO solvent and their potential as a drug carrier for oral administration. Fiber. Polym 2007, 8, 470–476. [Google Scholar]
- Mandal, BB; Kundu, SC. Self-assembled silk sericin/poloxamer nanoparticles as nanocarriers of hydrophobic and hydrophilic drugs for targeted delivery. Nanotechnology 2009, 20, 355101. [Google Scholar]
- Tsubouchi, K; Igarashi, Y; Takasu, Y; Yamada, H. Sericin enhances attachment of cultured human skin fibroblasts. Biosci. Biotechnol. Biochem 2005, 69, 403–405. [Google Scholar]
- Aramwit, P; Kanokpanont, S; Nakpheng, T; Srichana, T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci 2010, 11, 2200–2211. [Google Scholar]
- Teramoto, H; Kameda, T; Tamada, Y. Preparation of Gel film from Bombyx mori silk sericin and its characterization as a wound dressing. Biosci. Biotechnol. Biochem 2008, 72, 3189–3196. [Google Scholar]
- Mandal, BB; Priya, AS; Kundu, SC. Novel silk sericin/gelatin 3-D scaffolds and 2-D films: Fabrication and characterization for potential tissue engineering applications. Acta Biomater 2009, 5, 3007–3020. [Google Scholar]
- Tao, W; Li, MZ; Xie, RJ. Preparation and structure of porous silk sericin materials. Macromol. Mater. Eng 2005, 290, 188–194. [Google Scholar]
- Miyake, H; Wakisaka, H; Watanabe, T; Shimizu, Y; Nishikawa, S. Preparation of chitosan/sericin blend membrane and the characteristics of the resulting membrane. Sen-I Gakkaishi 2006, 62, 267–274. [Google Scholar]
- Ahmad, M; Benjakul, S. Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocolloids 2011, 25, 381–388. [Google Scholar]
- Lu, SZ; Wang, XQ; Lu, Q; Zhang, XH; Kluge, JA; Uppal, N; Omenetto, F; Kaplan, DL. Insoluble and flexible silk films with glycerol. Biomacromolecules 2010, 11, 143–150. [Google Scholar]
- Jackson, M; Mantsch, HH. Protein secondary structure from FT-IR spectroscopy: Correlation with dihedral angles from three-dimensional Ramachandran plots. Can. J. Chem 1991, 69, 1639–1642. [Google Scholar]
- Hu, X; Kaplan, D; Cebe, P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar]
- Lee, KG; Kweon, HY; Yeo, JH; Woo, SO; Lee, YW; Cho, CS; Kim, KH; Park, YH. Effect of methyl alcohol on the morphology and conformational characteristics of silk sericin. Int. J. Biol. Macromol 2003, 33, 75–80. [Google Scholar]
- Tsukada, M. Conformational analysis of silk sericin preliminary thermal treated in the presence of organic solvents or water. J. Seric. Sci. Jpn 1983, 52, 157–159. [Google Scholar]
- Konnerth, J; Stockel, F; Ulrich, M; Gindl, W. Elastic Properties of Adhesive Polymers. III. Adhesive Polymer Films Under Dry and Wet Conditions Characterized by Means of Nanoindentation. J. Appl. Polym. Sci 2010, 118, 1331–1334. [Google Scholar]
- Sothornvit, R; Olsen, CW; McHugh, TH; Krochta, JM. Tensile properties of compression-molded whey protein sheets: Determination of molding condition and glycerol-content effects and comparison with solution-cast films. J. Food Eng 2007, 78, 855–860. [Google Scholar]
- Jagadish, RS; Raj, B; Parameswara, P; Somashekar, R. Effect of glycerol on structure-property relations in chitosan/poly(ethylene oxide) blended films investigated using wide-angle X-ray diffraction. Polym. Int 2010, 59, 931–936. [Google Scholar]
- Zhang, HP; Yang, MY; Min, SJ; Feng, Q; Gao, X; Zhu, LJ. Preparation and characterization of a novel spongy hydrogel from aqueous Bombyx mori sericin. e-Polymers 2008, 66, 1–9. [Google Scholar]


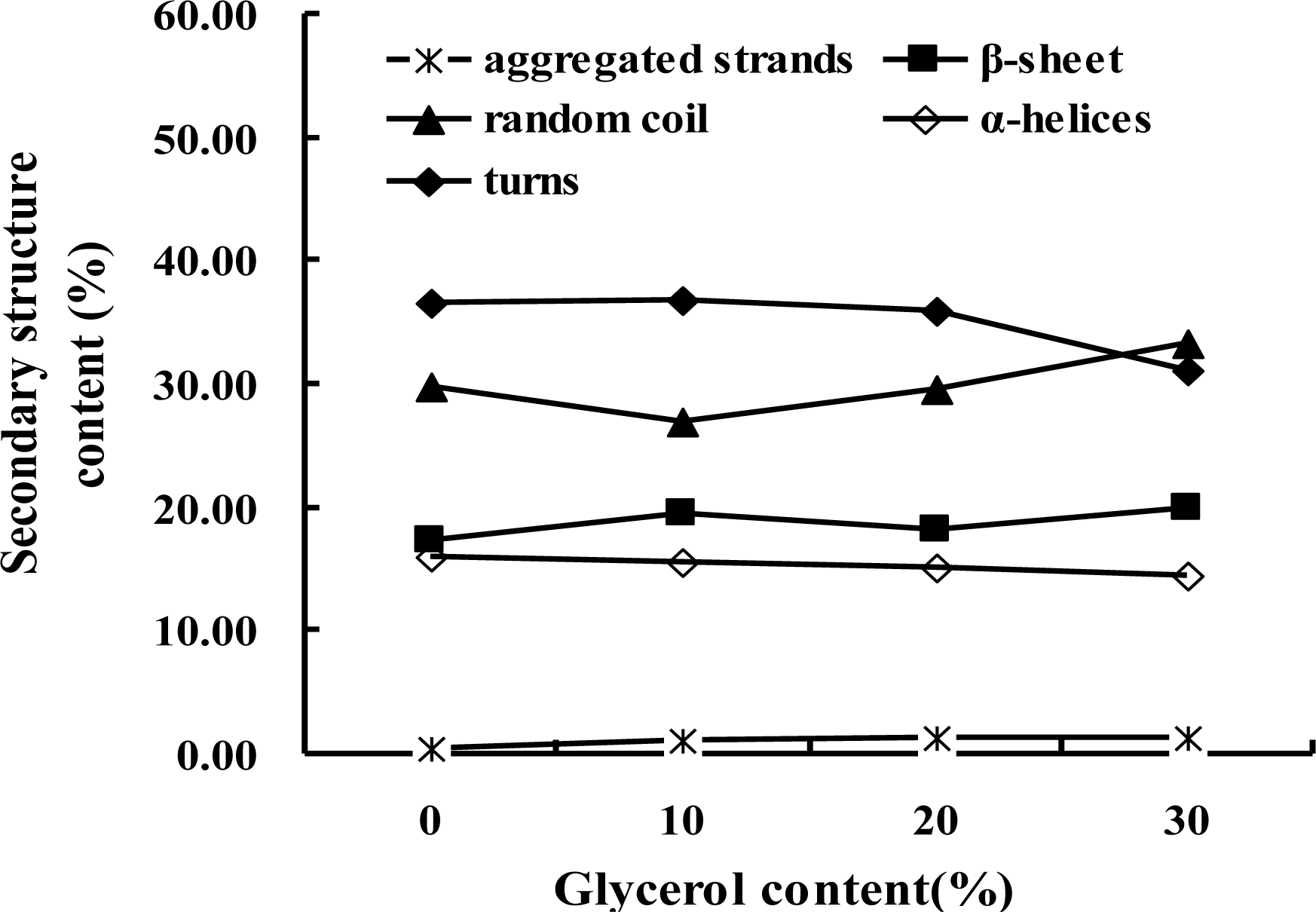



| Peaks | 1608 | 1617 | 1627 | 1638 | 1648 | 1658 | 1668 | 1679 | 1690 |
|---|---|---|---|---|---|---|---|---|---|
| Secondary Structure | Aggregated Strands | β-Sheet | Random Coil | α-Helices | Turns | ||||
| 0% Gly | 0.36 ± 0.05 | 17.19 ± 0.3 | 29.86 ± 2.4 | 15.91 ± 2.2 | 36.67 ± 0.1 | ||||
| 10% Gly | 1.15 ± 0.01 | 19.58 ± 0.2 | 26.99 ± 0.8 | 15.46 ± 1.2 | 36.81 ± 0.3 | ||||
| 20% Gly | 1.35 ± 0.02 | 18.08 ± 1.4 | 29.53 ± 0.7 | 15.16 ± 1.1 | 35.88 ± 1.7 | ||||
| 30% Gly | 1.25 ± 0.01 | 20.01 ± 2.0 | 33.28 ± 1.2 | 14.37 ± 1.3 | 31.09 ± 0.5 | ||||
| Glycerol Content (%) | Elastic Modulus (MPa) | Elongation at Break (%) | Tensile Strength (MPa) |
|---|---|---|---|
| 0 | 600.53 ±76.30 | 0.73 ±0.10 | 13.72 ±0.30 |
| 10 | 391.40 ±52.73 | 140.62 ±35.66 | 17.35 ±0.78 |
| 20 | 288.21 ±46.36 | 172.50 ±43.63 | 14.38 ±2.20 |
| 30 | 77.06 ±7.28 | 250.40 ±59.30 | 13.53 ±2.07 |
| 40 | 57.31 ±8.28 | 354.37 ±35.72 | 8.19 ±1.06 |
| Glycerol Content (%) | Elastic Modulus (MPa) | Elongation at Break (%) | Tensile Strength (MPa) |
|---|---|---|---|
| 0 | 0.64 ±0.09 | 53.58 ±8.69 | 0.21 ±0.05 |
| 10 | 4.08 ±0.53 | 130.37 ±29.67 | 0.73 ±0.09 |
| 20 | 3.70 ±0.45 | 168.24 ±31.47 | 1.15 ±0.07 |
| 30 | 2.68 ±0.32 | 244.67 ±51.70 | 1.12 ±0.08 |
| 40 | 2.26 ±0.26 | 271.33 ±42.51 | 1.18 ±0.08 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, H.; Deng, L.; Yang, M.; Min, S.; Yang, L.; Zhu, L. Enhancing Effect of Glycerol on the Tensile Properties of Bombyx mori Cocoon Sericin Films. Int. J. Mol. Sci. 2011, 12, 3170-3181. https://doi.org/10.3390/ijms12053170
Zhang H, Deng L, Yang M, Min S, Yang L, Zhu L. Enhancing Effect of Glycerol on the Tensile Properties of Bombyx mori Cocoon Sericin Films. International Journal of Molecular Sciences. 2011; 12(5):3170-3181. https://doi.org/10.3390/ijms12053170
Chicago/Turabian StyleZhang, Haiping, Lianxia Deng, Mingying Yang, Sijia Min, Lei Yang, and Liangjun Zhu. 2011. "Enhancing Effect of Glycerol on the Tensile Properties of Bombyx mori Cocoon Sericin Films" International Journal of Molecular Sciences 12, no. 5: 3170-3181. https://doi.org/10.3390/ijms12053170





