How Does the Preparation of Rye Porridge Affect Molecular Weight Distribution of Extractable Dietary Fibers?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Content of Dietary Fiber and Its Components
2.2. Molecular Weight Distribution of DF Components
2.2.1. Molecular Weight Distribution of Fructan
2.2.2. Molecular Weight Distributions of Arabinoxylan
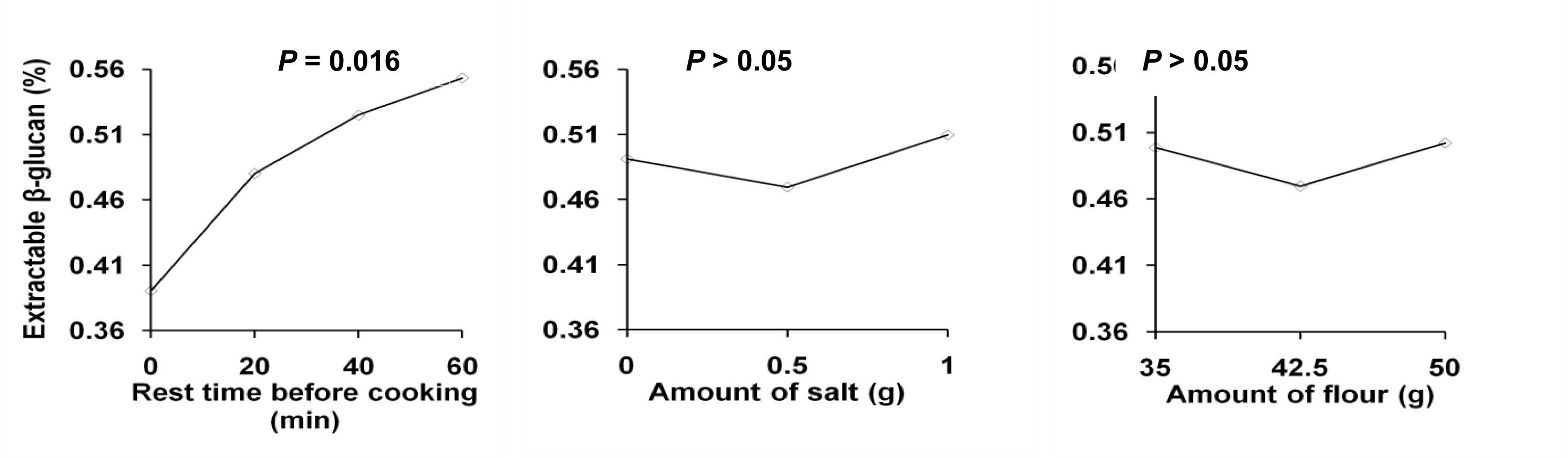
2.2.3. Molecular Weight of Extractable β-Glucan
3. Experimental Section
3.1. Materials
3.2. Chemicals and Reagents
3.3. Experiment 1
3.3.1. Design of Experiment 1
3.3.2. Porridge Making
3.4. Experiment 2
3.5. Analytical Methods
3.6. Statistical Analyses
4. Conclusions
Acknowledgments
References
- Åman, P; Andersson, AAM; Rakha, A; Andersson, R. Rye, a healthy cereal full of dietary fiber. Cereal Foods World 2010, 55, 231–234. [Google Scholar]
- Andersson, R; Fransson, G; Tietjen, M; Åman, P. Content and molecular-weight distribution of dietary fiber components in whole-grain rye flour and bread. J. Agric. Food Chem 2009, 57, 2004–2008. [Google Scholar]
- Hansen, HB; Rasmussen, CV; Knudsen, KEB; Hansen, Å. Effects of genotype and harvest year on content and composition of dietary fibre in rye (Secale cereale L.) grain. J. Sci. Food Agric 2003, 83, 76–85. [Google Scholar]
- Vinkx, CJA; Delcour, JA. Rye (Secale cereale L.) arabinoxylans: A critical review. J. Cereal Sci 1996, 24, 1–14. [Google Scholar]
- Wood, PJ; Weisz, J; Blackwell, BA. Structural studies of (1→3),(1→4)-β-d-glucans by 13C-nuclear magnetic resonance spectroscopy and by rapid analysis of cellulose-like regions using high-performance anion-exchange chromatography of oligosaccharides released by lichenase. Cereal Chem 1994, 71, 301–307. [Google Scholar]
- Roberfroid, MB. Introducing inulin-type fructans. Br. J. Nutr 2005, 93, S13–S25. [Google Scholar]
- Bornet, FRJ. Fructo-oligosaccharides and other fructans: Chemistry, structure and nutritional effects. In Advanced Dietary Fibre Technology; McCleary, BV, Prosky, L, Eds.; Wiley-Blackwell: Chichester, UK, 2008; pp. 480–493. [Google Scholar]
- EFSA (European Food Safety Authority) Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Carbohydrates and Dietary Fibre. EFSA J 2010, 8. [Google Scholar] [CrossRef]
- National Nutrient Database for Standard Reference, SR 23. Available online: http://www.nal.usda.gov/fnic/foodcomp/search/ (accessed on 15 December 2010).
- Buttriss, JL; Stokes, CS. Dietary fibre and health: An overview. Nutr. Bull 2008, 33, 186–200. [Google Scholar]
- Kendall, CWC; Esfahani, A; Jenkins, DJA. The link between dietary fibre and human health. Food Hydrocoll 2010, 24, 42–48. [Google Scholar]
- Brennan, CS; Cleary, LJ. The potential use of cereal (1→3,1→4)-β-d-glucans as functional food ingredients. J. Cereal Sci 2005, 42, 1–13. [Google Scholar]
- Regand, A; Tosh, SM; Wolever, TMS; Wood, PJ. Physicochemical properties of beta-glucan in differently processed oat foods influence glycemic response. J. Agric. Food Chem 2009, 57, 8831–8838. [Google Scholar]
- Åman, P; Rimsten, L; Andersson, R. Molecular weight distribution of beta-glucan in oat-based foods. Cereal Chem 2004, 81, 356–360. [Google Scholar]
- Andersson, AAM; Armö, E; Grangeon, E; Fredriksson, H; Andersson, R; Åman, P. Molecular weight and structure units of (1→3, 1→4)-β-glucans in dough and bread made from hull-less barley milling fractions. J. Cereal Sci 2004, 40, 195–204. [Google Scholar]
- EFSA (European Food Safety Authority) Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Beta-Glucans and Maintenance of Normal Blood Cholesterol Concentrations (ID 754,755,757,801,1465,2934) and Maintenance or Achievement of a Normal Body Weight (ID 820,823) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006 on Request from the European Commission. EFSA J 2009, 7. [Google Scholar] [CrossRef]
- Dornez, E; Cuyvers, S; Gebruers, K; Delcour, JA; Courtin, CM. Contribution of wheat endogenous and wheat kernel associated microbial endoxylanases to changes in the arabinoxylan population during breadmaking. J. Agric. Food Chem 2008, 56, 2246–2253. [Google Scholar]
- Rasmussen, CV; Hansen, HB; Hansen, Å; Larsen, LM. pH-, temperature- and time-dependent activities of endogenous endo-[beta]-d-xylanase, [beta]-d-xylosidase and [alpha]-l-arabinofuranosidase in extracts from ungerminated rye (Secale cereale L.) grain. J. Cereal Sci 2001, 34, 49–60. [Google Scholar]
- Poutanen, K. Enzymes: An important tool in the improvement of the quality of cereal foods. Trends Food Sci. Technol 1997, 8, 300–306. [Google Scholar]
- Salmenkallio-Marttila, M; Hovinen, S. Enzyme activities, dietary fibre components and rheological properties of wholemeal flours from rye cultivars grown in Finland. J. Sci. Food Agric 2005, 85, 1350–1356. [Google Scholar]
- Englyst, HN; Macfarlane, GT. Breakdown of resistant and readily digestible starch by human gut bacteria. J. Sci. Food Agric 1986, 37, 699–706. [Google Scholar]
- Andersson, AAM; Rüegg, N; Åman, P. Molecular weight distribution and content of water-extractable beta-glucan in rye crisp bread. J. Cereal Sci 2008, 47, 399–406. [Google Scholar]
- Laurikainen, T; Härkönen, H; Autio, K; Poutanen, K. Effects of enzymes in fibre-enriched baking. J. Sci. Food Agric 1998, 76, 239–249. [Google Scholar]
- Kawakami, A; Yoshida, M; van den Ende, W. Molecular cloning and functional analysis of a novel 6 & 1-FEH from wheat (Triticum aestivum L.) preferentially degrading small graminans like bifurcose. Gene 2005, 358, 93–101. [Google Scholar]
- Chalmers, J; Lidgett, A; Cummings, N; Cao, Y; Forster, J; Spangenberg, G. Molecular genetics of fructan metabolism in perennial ryegrass. Plant Biotechnol. J 2005, 3, 459–474. [Google Scholar]
- van den Ende, W; Clerens, S; Vergauwen, R; van Riet, L; van Laere, A; Yoshida, M; Kawakami, A. Fructan 1-exohydrolases: β-(2,1)-trimmers during graminan biosynthesis in stems of wheat Purification, characterization, mass mapping, and cloning of two fructan 1-exohydrolase isoforms. Plant Physiol 2003, 131, 621–631. [Google Scholar]
- Marx, SP; Nösberger, J; Frehner, M. Seasonal variation of fructan-β-fructosidase (FEH) activity and characterization of a β-(2-1)-linkage specific FEH from tubers of Jerusalem artichoke (Helianthus tuberosus). New Phytol 1997, 135, 267–277. [Google Scholar]
- Rakha, A; Åman, P; Andersson, R. Characterisation of dietary fibre components in rye products. Food Chem 2010, 119, 859–867. [Google Scholar]
- Cauvain, SP. Breadmaking: An overview. In Bread Making: Improving Quality; CRC Press: Boca Raton, FL, USA, 2003; pp. 8–28. [Google Scholar]
- Hui, YH; Meunier-Goddik, L; Hansen, ÅS; Josephsen, J; Nip, W-K; Stanfield, PS; Toldra, F (Eds.) Handbook of Food and Beverage Fermentation Technology; CRC Press, Taylor & Francis Group: London, UK, 2004; pp. 840–871.
- Cleemput, G; Hessing, M; van Oort, M; Deconynck, M; Delcour, JA. Purification and characterization of a [beta]-d-xylosidase and an endo-xylanase from wheat flour. Plant Physiol 1997, 113, 377–386. [Google Scholar]
- Meyer, AS; Rosgaard, L; Sørensen, HR. The minimal enzyme cocktail concept for biomass processing. J. Cereal Sci 2009, 50, 337–344. [Google Scholar]
- Katina, K; Liukkonen, KH; Kaukovirta-Norja, A; Adlercreutz, H; Heinonen, SM; Lampi, AM; Pihlava, JM; Poutanen, K. Fermentation-induced changes in the nutritional value of native or germinated rye. J Cereal Sci 2007, 46, 348–355. [Google Scholar]
- Debyser, W; Peumans, WJ; Van Damme, EJM; Delcour, JA. Triticum aestivum xylanase inhibitor (TAXI), a new class of enzyme inhibitor affecting breadmaking performance. J Cereal Sci 1999, 30, 39–43. [Google Scholar]
- Goesaert, H; Elliott, G; Kroon, PA; Gebruers, K; Courtin, CM; Robben, J; Delcour, JA; Juge, N. Occurrence of proteinaceous endoxylanase inhibitors in cereals. Biochim. Biophys. Acta Proteins Proteomics 2004, 1696, 193–202. [Google Scholar]
- Beer, MU; Wood, PJ; Weisz, J; Fillion, N. Effect of cooking and storage on the amount and molecular weight of (1→3)(1→4)-β-d-glucan extracted from oat products by an in vitro digestion system. Cereal Chem 1997, 74, 705–709. [Google Scholar]
- Rimsten, L; Stenberg, T; Andersson, R; Andersson, A; Åman, P. Determination of beta-glucan molecular weight using SEC with Calcofluor detection in cereal extracts. Cereal Chem 2003, 80, 485–490. [Google Scholar]
- Theander, O; Åman, P; Westerlund, E; Andersson, R; Pettersson, D. Total dietary fiber determined as neutral sugar residues, uronic acid residues, and Klason lignin (the Uppsala method): Collaborative study. J. AOAC Int 1995, 78, 1030–1044. [Google Scholar]
- Andersson, AAM; Merker, A; Nilsson, P; Sørensen, H; Åman, P. Chemical composition of the potential new oilseed crops Barbarea vulgaris, Barbarea verna and Lepidium campestre. J. Sci. Food Agric 1999, 79, 179–186. [Google Scholar]
- Loosveld, AMA; Grobet, PJ; Delcour, JA. Contents and structural features of water-extractable arabinogalactan in wheat flour fractions. J. Agric. Food Chem 1997, 45, 1998–2002. [Google Scholar]
- McCleary, BV; Murphy, A; Mugford, DC. Determination of oligofructans and fructan polysaccharides in foodstuffs by an enzymatic/spectrophotometric method: Collaborative study. J. AOAC Int 1997, 83, 356–364. [Google Scholar]
- McCleary, BV; Codd, R. Measurement of (1-3)(1-4)-β-d-glucan in barley and oats: A streamlined enzymic procedure. J. Sci. Food Agric 1991, 55, 303–312. [Google Scholar]

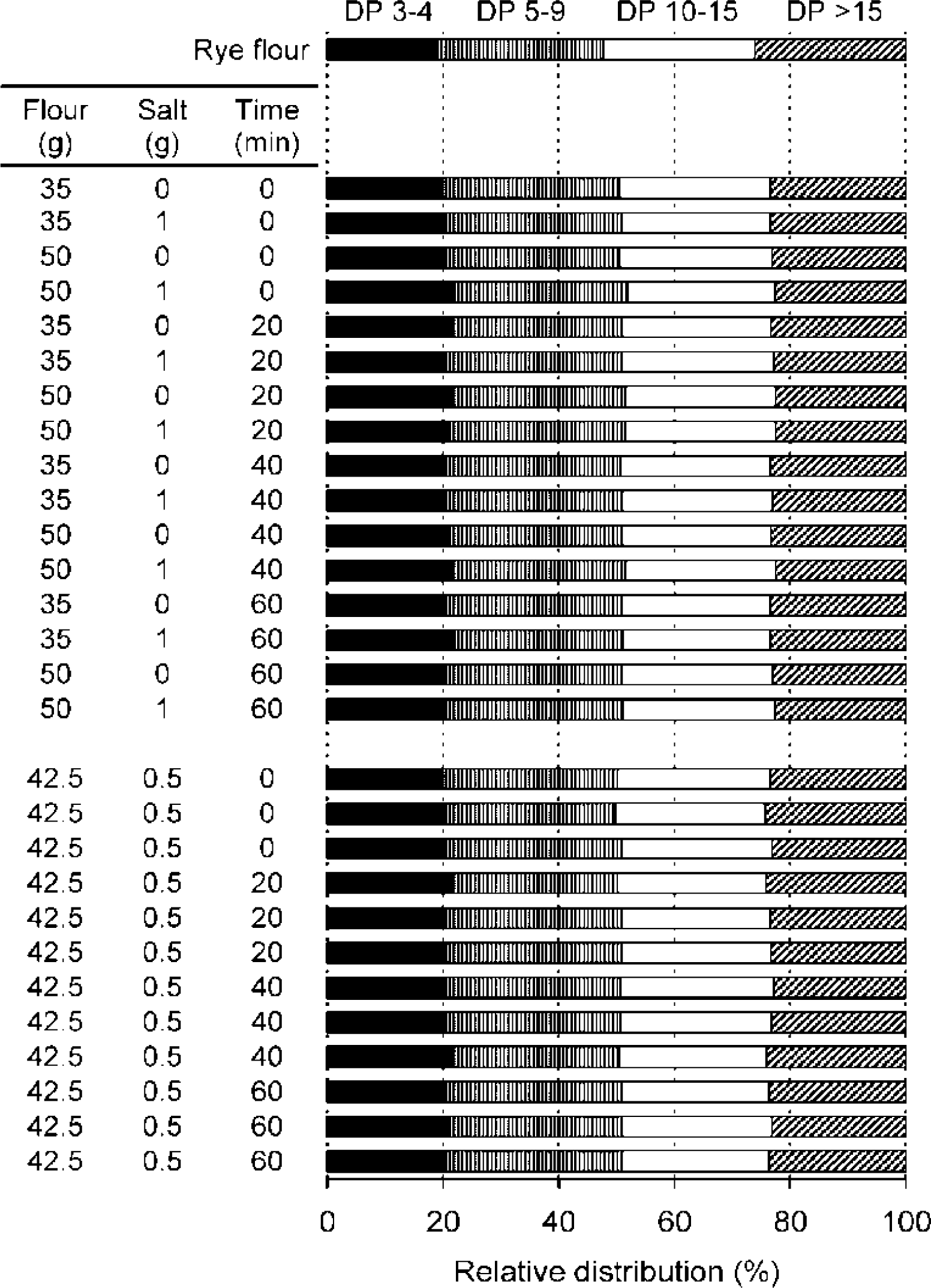



 flour, —— 0 min, – - – - – 20 min, ------- 40 min, _ _ _ _ 60 min).
flour, —— 0 min, – - – - – 20 min, ------- 40 min, _ _ _ _ 60 min).
 flour, —— 0 min, – - – - – 20 min, ------- 40 min, _ _ _ _ 60 min).
flour, —— 0 min, – - – - – 20 min, ------- 40 min, _ _ _ _ 60 min).
| Sample | DF 2 | Arabinoxylan 3 | β-Glucan | Fructan | |||
|---|---|---|---|---|---|---|---|
| Total | Extractable | Total | Extractable | Total | Extractable | Extractable | |
| Flour | 20.2 | 7.5 | 8.2 | 2.5 | 2.2 | 0.31 | 4.2 |
| Porridge, without rest time | 23.2 | 7.3 | 8.6 | 2.5 | 2.3 | 0.39 | 3.9 |
| Porridge, with 60 min rest time | 23.5 | 7.7 | 8.6 | 2.6 | 2.3 | 0.55 | 4.1 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rakha, A.; Åman, P.; Andersson, R. How Does the Preparation of Rye Porridge Affect Molecular Weight Distribution of Extractable Dietary Fibers? Int. J. Mol. Sci. 2011, 12, 3381-3393. https://doi.org/10.3390/ijms12053381
Rakha A, Åman P, Andersson R. How Does the Preparation of Rye Porridge Affect Molecular Weight Distribution of Extractable Dietary Fibers? International Journal of Molecular Sciences. 2011; 12(5):3381-3393. https://doi.org/10.3390/ijms12053381
Chicago/Turabian StyleRakha, Allah, Per Åman, and Roger Andersson. 2011. "How Does the Preparation of Rye Porridge Affect Molecular Weight Distribution of Extractable Dietary Fibers?" International Journal of Molecular Sciences 12, no. 5: 3381-3393. https://doi.org/10.3390/ijms12053381




