Origin of Evolution versus Origin of Life: A Shift of Paradigm
Abstract
:1. Introduction
2. The Primordial Ancestor and the Conditions for Evolution
2.1. What Is the Question?
2.2. Three Conditions for Evolution
2.3. Two Schools of Thought
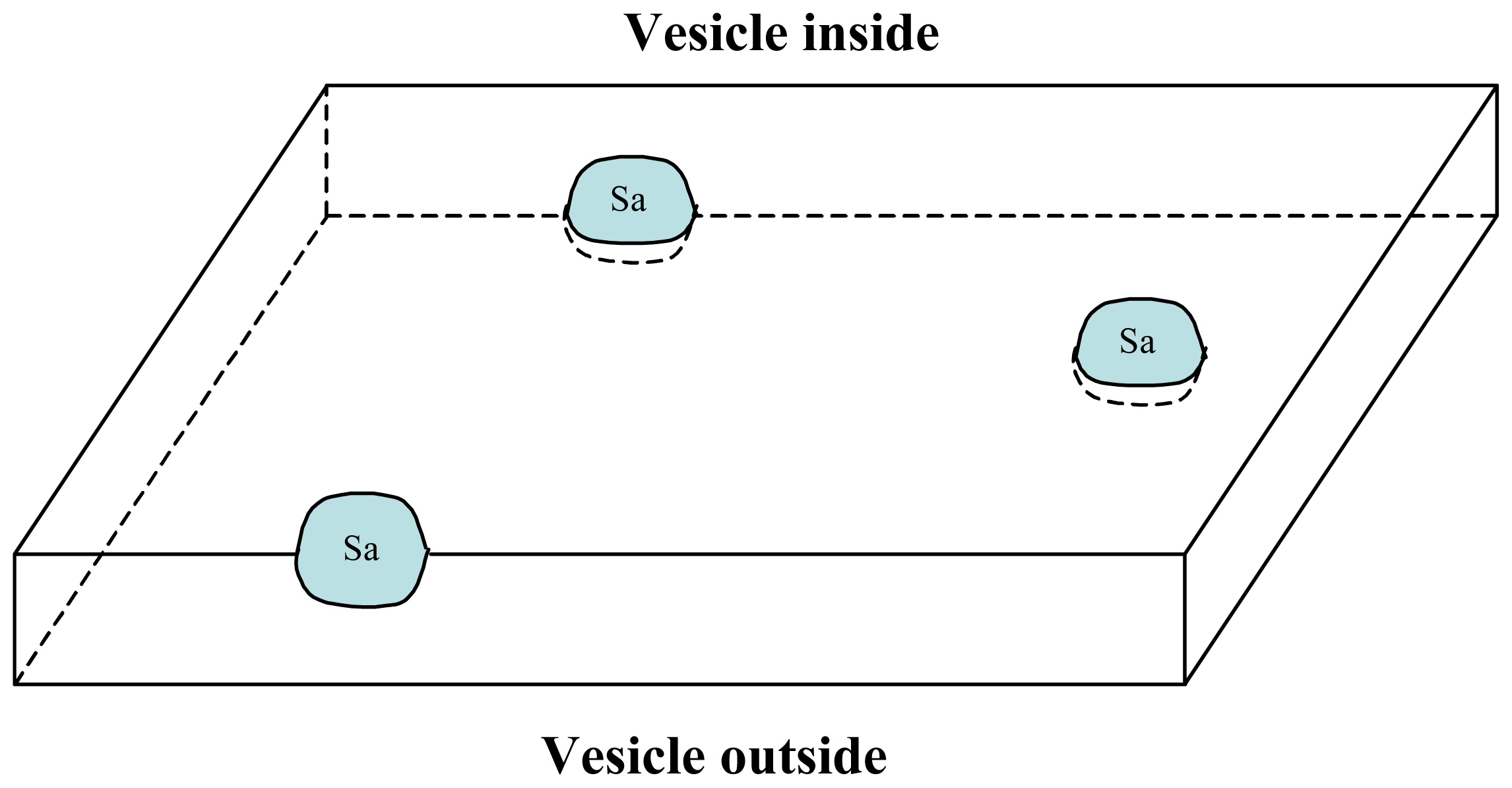
3. A Plausible Solution within a Lipidic Vesicle-Based Model
3.1. Proposal Model
3.2. Consequences of the Model: Homochirality and Polymers
3.3. Next Experiments
4. The Constraints of Lipidic Vesicle-Based Models
4.1. Need for Lost City-Like Hydrothermal Vents
4.2. Synthesis of Lipid Compounds
4.3. The Phosphorus Issue
4.4. Need for a Tectonic Plate Dynamic Regime?
4.5. Origin of Evolution versus Origin of Life: Consequences
5. Conclusions
Acknowledgments
References
- Tsokolov, SA. Why is the definition of life so elusive? Epistemological considerations. Astrobiology 2009, 9, 401–412. [Google Scholar]
- Gayon, J. Defining life: Synthesis and conclusions. Orig. Life Evol. Biosph 2010, 40, 231–244. [Google Scholar]
- Luisi, PL. The Emergence of Life: From Chemical Origins to Synthetic Biology; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Skar, J. Introduction: Self-organization as an actual theme. Philos. Transact. A Math. Phys. Eng. Sci 2003, 361, 1049–1056. [Google Scholar]
- Coveney, PV. Self-organization and complexity: A new age for theory, computation and experiment. Philos. Transact. A Math. Phys. Eng. Sci 2003, 361, 1057–1079. [Google Scholar]
- Schrödinger, E. What Is Life? With Mind and Matter and Autobiographical Sketches; Cambridge University Press: Cambridge, NY, USA, 2000. [Google Scholar]
- Bergé, P; Pomeau, Y; Vidal, C. L’ordre dans le chaos; Edition Hermann: Paris, France, 1988. [Google Scholar]
- Andrade, E. From external to internal measurement: A form theory approach to evolution. Biosystems 2000, 57, 49–62. [Google Scholar]
- Tessera, M. Life began when evolution began: A lipidic vesicle-based scenario. Orig. Life Evol. Biosph 2009, 39, 559–564. [Google Scholar]
- Lazcano, A. Which way to life? Orig. Life Evol. Biosph 2010, 40, 161–167. [Google Scholar]
- Oyama, S; Griffiths, PE; Gray, RD. Cycles of Contingency: Developmental Systems and Evolution; MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Segre, D; Ben-Eli, D; Lancet, D. Compositional genomes: Prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl. Acad. Sci. USA 2000, 97, 4112–4117. [Google Scholar]
- Vasas, V; Szathmary, E; Santos, M. Lack of evolvability in self-sustaining autocatalytic networks constraints metabolism-first scenarios for the origin of life. Proc. Natl. Acad. Sci. USA 2010, 107, 1470–1475. [Google Scholar]
- Rasi, S; Mavelli, F; Luisi, PL. Matrix effect in oleate micelles-vesicles transformation. Orig. Life Evol. Biosph 2004, 34, 215–224. [Google Scholar]
- Luisi, PL; Stano, P; Rasi, S; Mavelli, F. A possible route to prebiotic vesicle reproduction. Artif. Life 2004, 10, 297–308. [Google Scholar]
- Walde, P. Surfactant assemblies and their various possible roles for the origin(s) of life. Orig. Life Evol. Biosph 2006, 36, 109–150. [Google Scholar]
- Weber, BH. What is life? Defining life in the context of emergent complexity. Orig. Life Evol. Biosph 2010, 40, 221–229. [Google Scholar]
- Weber, AL; Pizzarello, S. The peptide-catalyzed stereospecific synthesis of tetroses: A possible model for prebiotic molecular evolution. Proc. Natl. Acad. Sci. USA 2006, 103, 12713–12717. [Google Scholar]
- Mielke, RE; Russell, MJ; Wilson, PR; McGlynn, SE; Coleman, M; Kidd, R; Kanik, I. Design, fabrication, and test of a hydrothermal reactor for origin-of-life experiments. Astrobiology 2010, 10, 799–810. [Google Scholar]
- Mansy, SS; Schrum, JP; Krishnamurthy, M; Tobe, S; Treco, DA; Szostak, JW. Template-directed synthesis of a genetic polymer in a model protocell. Nature 2008, 454, 122–125. [Google Scholar]
- Kelley, DS; Karson, JA; Fruh-Green, GL; Yoerger, DR; Shank, TM; Butterfield, DA; Hayes, JM; Schrenk, MO; Olson, EJ; Proskurowski, G; Jakuba, M; Bradley, A; Larson, B; Ludwig, K; Glickson, D; Buckman, K; Bradley, AS; Brazelton, WJ; Roe, K; Elend, MJ; Delacour, A; Bernasconi, SM; Lilley, MD; Baross, JA; Summons, RE; Sylva, SP. A serpentinite-hosted ecosystem: The Lost City hydrothermal field. Science 2005, 307, 1428–1434. [Google Scholar]
- Nisbet, EG; Sleep, NH. The habitat and nature of early life. Nature 2001, 409, 1083–1091. [Google Scholar]
- Hagmann, M. Gunter wachtershauser profile. Between a rock and a hard place. Science 2002, 295, 2006–2007. [Google Scholar]
- Brasier, MD; Green, OR; Jephcoat, AP; Kleppe, AK; van Kranendonk, MJ; Lindsay, JF; Steele, A; Grassineau, NV. Questioning the evidence for Earth’s oldest fossils. Nature 2002, 416, 76–81. [Google Scholar]
- Proskurowski, G; Lilley, MD; Seewald, JS; Fruh-Green, GL; Olson, EJ; Lupton, JE; Sylva, SP; Kelley, DS. Abiogenic hydrocarbon production at lost city hydrothermal field. Science 2008, 319, 604–607. [Google Scholar]
- Russell, MJ; Hall, AJ; Martin, W. Serpentinization as a source of energy at the origin of life. Geobiology 2010, 8, 355–371. [Google Scholar]
- Ludwig, KA; Kelley, DS; Butterfield, DA; Nelson, BK; Früh-Green, G. Formation and evolution of carbonate chimneys at the Lost City Hydrothermal Field. Geochim. Cosmochim. Acta 2011, 70, 3625–3645. [Google Scholar]
- Baross, J; Hoffman, SE. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biosph 1985, 15, 327–345. [Google Scholar]
- Rushdi, AI; Simoneit, BR. Lipid formation by aqueous Fischer-Tropsch-type synthesis over a temperature range of 100 to 400 degrees C. Orig. Life Evol. Biosph 2001, 31, 103–118. [Google Scholar]
- Martin, W; Baross, J; Kelley, D; Russell, MJ. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol 2008, 6, 805–814. [Google Scholar]
- Konn, C; Charlou, JL; Donval, JP; Holm, NG; Dehairs, F; Bouillon, S. Hydrocarbons and oxidized organic compounds in hydrothermal fluids from Rainbow and Lost City ultramafic-hosted vents. Chem. Geol 2009, 258, 299–314. [Google Scholar] [Green Version]
- Amend, JP; McCollom, TM. Zaikowski, L, Ed.; Energetics of biomolecule synthesis on early earth. In Chemical Evolution II: From the Origins of Life to Modern Societyl; ACS Symposium Series, American Chemical Society: Washington, DC, USA, 2009. [Google Scholar]
- Baaske, P; Weinert, FM; Duhr, S; Lemke, KH; Russell, MJ; Braun, D. Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc. Natl. Acad. Sci. USA 2007, 104, 9346–9351. [Google Scholar]
- Budin, I; Bruckner, RJ; Szostak, JW. Formation of protocell-like vesicles in a thermal diffusion column. J. Am. Chem. Soc 2009, 131, 9628–9629. [Google Scholar]
- Deamer, D; Dworkin, JP; Sandford, SA; Bernstein, MP; Allamandola, LJ. The first cell membranes. Astrobiology 2002, 2, 371–381. [Google Scholar]
- Maurer, SE; Deamer, DW; Boncella, JM; Monnard, PA. Chemical evolution of amphiphiles: Glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology 2009, 9, 979–987. [Google Scholar]
- Namani, T; Deamer, DW. Stability of model membranes in extreme environments. Orig. Life Evol. Biosph 2008, 38, 329–341. [Google Scholar]
- Simoneit, BRT; Rushdi, AI; Deamer, DW. Abiotic formation of acylglycerols under simulated hydrothermal conditions and self-assembly properties of such lipid products. Adv. Space Res 2007, 40, 1649–1656. [Google Scholar]
- Albarede, F; Blichert-Toft, J. Gerin, M, Maurel, MC, Eds.; The terrestrial cradle of life. In Origins of Life: Self-Organization and/or Biological Evolution? EDP Sciences: Paris, France, 2009; pp. 1–12. [Google Scholar]
- Lane, N; Allen, JF; Martin, W. How did LUCA make a living? Chemiosmosis in the origin of life. Bioessays 2010, 32, 271–280. [Google Scholar]
- Wolfe-Simon, F; Blum, JS; Kulp, TR; Gordon, GW; Hoeft, SE; Pett-Ridge, J; Stolz, JF; Webb, SM; Weber, PK; Davies, PC; Anbar, AD; Oremland, RS. A bacterium that can grow by using arsenic instead of phosphorus. Science 2010. [Google Scholar]
- Schwartz, AW. Arsenate DNA—Evidence for a vital force? Orig. Life Evol. Biosph 2011, 41, 1. [Google Scholar]
- Silver, S; Phung, LT. Novel expansion of living chemistry or just a serious mistake? FEMS Microbiol. Lett 2011, 315, 79–80. [Google Scholar]
- Brack, A; Horneck, G; Cockell, CS; Berces, A; Belisheva, NK; Eiroa, C; Henning, T; Herbst, T; Kaltenegger, L; Leger, A; Liseau, R; Lammer, H; Selsis, F; Beichman, C; Danchi, W; Fridlund, M; Lunine, J; Paresce, F; Penny, A; Quirrenbach, A; Rottgering, H; Schneider, J; Stam, D; Tinetti, G; White, GJ. Origin and evolution of life on terrestrial planets. Astrobiology 2010, 10, 69–76. [Google Scholar]
- O’Neill, C; Jellinek, AM; Lenardic, A. Conditions for the onset of plate tectonics on terrestrial planets moons. Earth Planet. Sci. Lett 2007, 261, 20–32. [Google Scholar]
- Albarede, F. Volatile accretion history of the terrestrial planets and dynamic implications. Nature 2009, 461, 1227–1233. [Google Scholar]
- Morris, RV; Ruff, SW; Gellert, R; Ming, DW; Arvidson, RE; Clark, BC; Golden, DC; Siebach, K; Klingelhofer, G; Schroder, C; Fleischer, I; Yen, AS; Squyres, SW. Identification of carbonate-rich outcrops on Mars by the Spirit rover. Science 2010, 329, 421–424. [Google Scholar]
- Shapiro, R; Schulze-Makuch, D. The search for alien life in our solar system: Strategies and priorities. Astrobiology 2009, 9, 335–343. [Google Scholar]
- Vance, S; Harnmeijer, J; Kimura, J; Hussmann, H; Demartin, B; Brown, JM. Hydrothermal systems in small ocean planets. Astrobiology 2007, 7, 987–1005. [Google Scholar]
- Nowak, MA; Ohtsuki, H. Prevolutionary dynamics and the origin of evolution. Proc. Natl. Acad. Sci. USA 2008, 105, 14924–14927. [Google Scholar]








© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tessera, M. Origin of Evolution versus Origin of Life: A Shift of Paradigm. Int. J. Mol. Sci. 2011, 12, 3445-3458. https://doi.org/10.3390/ijms12063445
Tessera M. Origin of Evolution versus Origin of Life: A Shift of Paradigm. International Journal of Molecular Sciences. 2011; 12(6):3445-3458. https://doi.org/10.3390/ijms12063445
Chicago/Turabian StyleTessera, Marc. 2011. "Origin of Evolution versus Origin of Life: A Shift of Paradigm" International Journal of Molecular Sciences 12, no. 6: 3445-3458. https://doi.org/10.3390/ijms12063445



