Differences in Soil Properties and Bacterial Communities between the Rhizosphere and Bulk Soil and among Different Production Areas of the Medicinal Plant Fritillaria thunbergii
Abstract
:1. Introduction
2. Results
2.1. pH, OM and the Activities of Urease and Phosphatase in Soil
2.2. Element Concentration in Soil
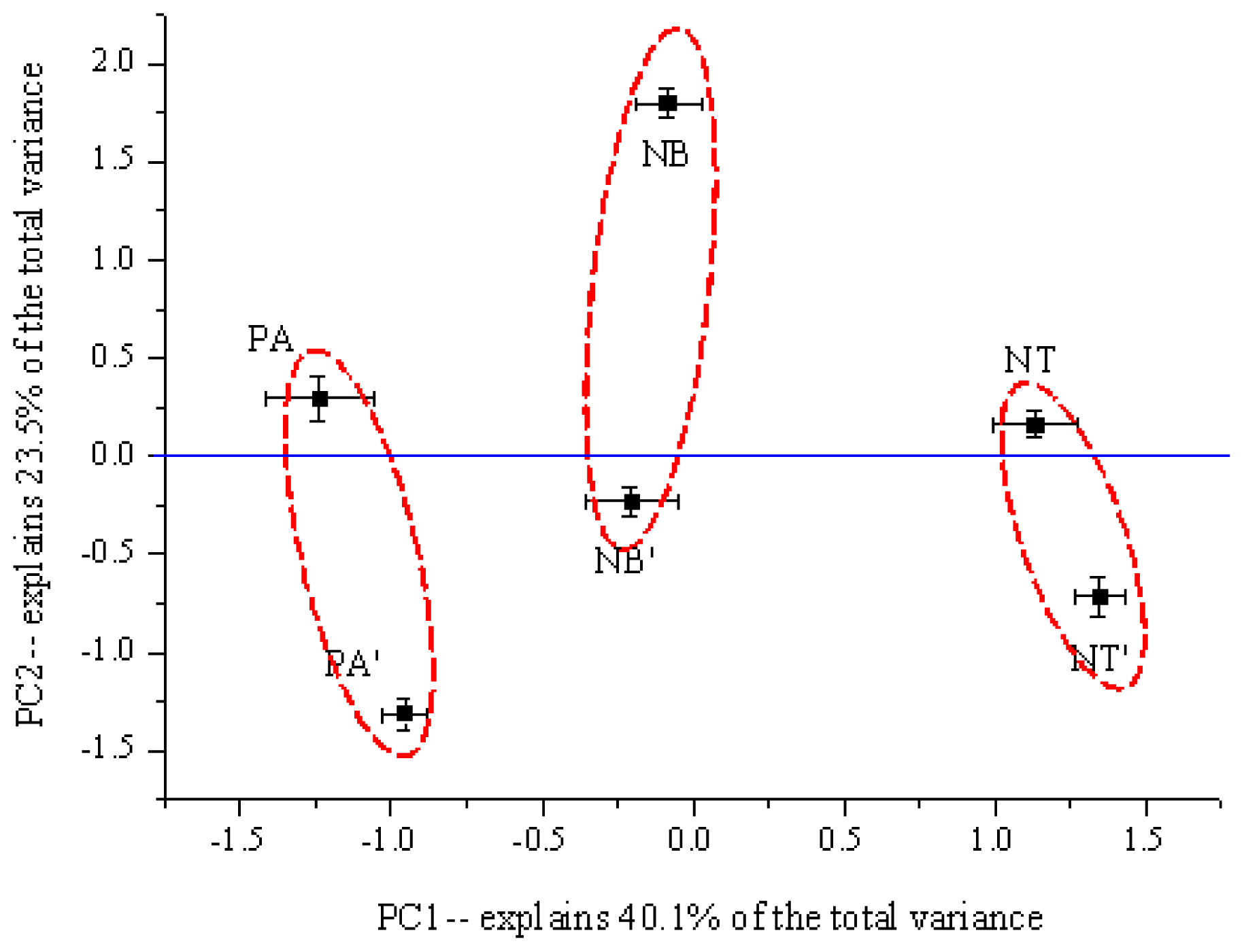
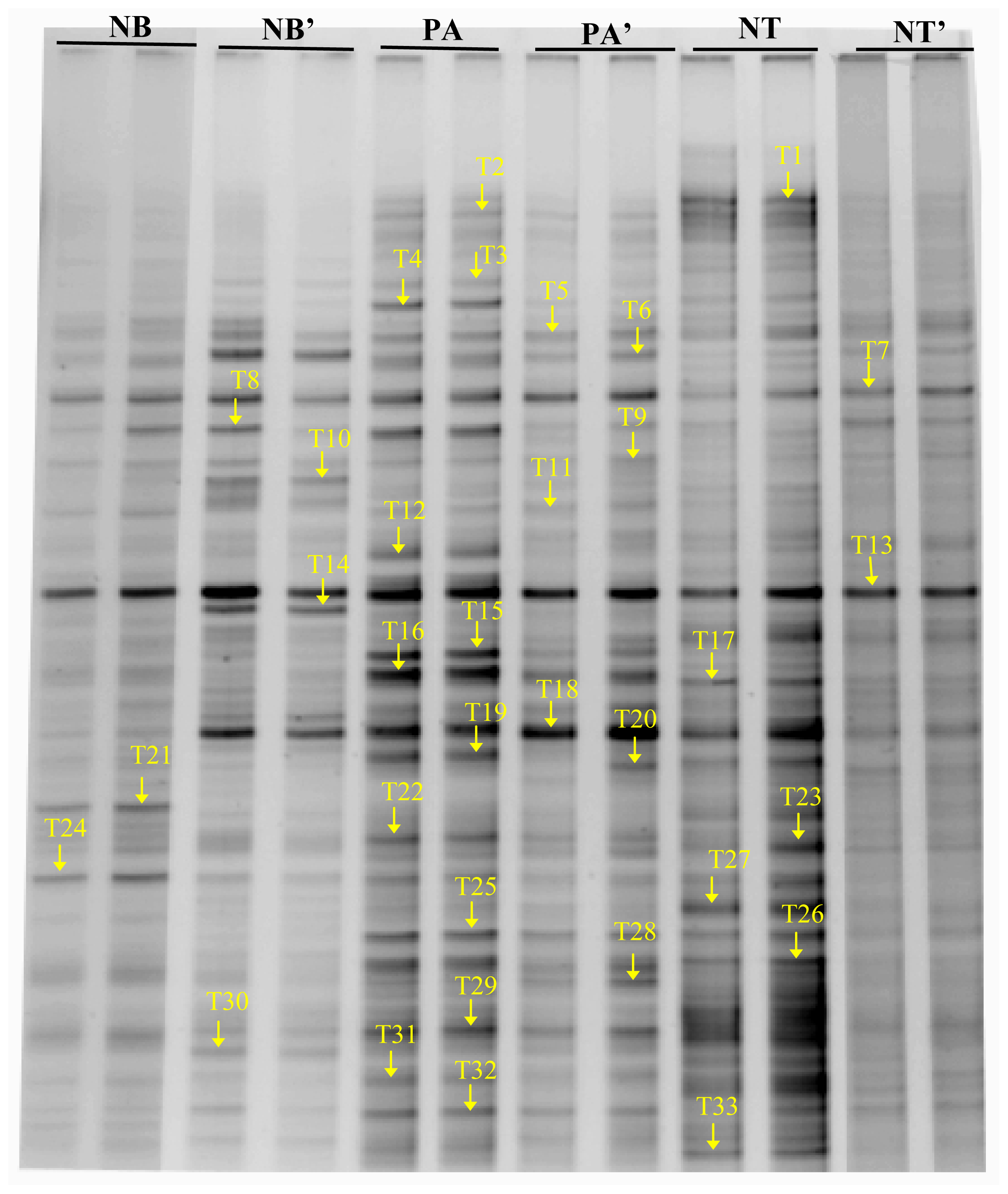
2.3. The Structure of Bacterial Community in Soil
3. Discussion
4. Experimental Section
4.1. Soil Sample Collection
4.2. pH, OM, the Activities of Urease and Phosphatase in Soil
4.3. Analysis of the Bacterial Community Composition by PCR-DGGE
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
References
- Huang, LQ; Guo, LP; Hu, J; Shao, AJ. Molecular mechanism and genetic basis of geoherbs. China J. Chin. Mater. Med 2008, 33, 2303–2308. [Google Scholar]
- Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia; Chemical Industry Press: Beijing, China, 2005.
- Jiang, S; Duan, JA; Qian, DW; Yan, H; Yu, G. Effects of microbes in plant rhizosphere on geoherbalism. Soils 2009, 41, 344–349. [Google Scholar]
- Xiao, XH; Chen, SL; Huang, LQ; Xiao, PG. Survey of investigations on Dao-di Chinese medicinal materials in China since 1980s. China J. Chin. Mater. Med 2009, 34, 519–523. [Google Scholar]
- Wang, Y; Wei, JH; Chen, SL; Sun, CZ; Zhao, RH; Liu, ZQ; Xiao, XH; Wang, JY; Zhou, YQ. Suitability evaluation of Fritillaria thunbergii’s producing area based on GIS. Mod. Chin. Med 2006, 8, 4–7. [Google Scholar]
- Berg, G; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol 2009, 68, 1–13. [Google Scholar]
- Bürgmann, H; Meier, S; Bunge, M; Widmer, F; Zeyer, J. Effects of model root exudates on structure and activity of a soil diazotroph community. Environ. Microbiol 2005, 7, 1711–1724. [Google Scholar]
- Raaijmakers, JM; Paulitz, CT; Steinberg, C; Alabouvette, C; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar]
- Karthikeyan, B; Jaleel, CA; Lakshmanan, GM; Deiveekasundaram, M. Studies on rhizosphere microbial diversity of some commercially important medicinal plants. Colloids Surf. B Biointerfaces 2008, 62, 143–145. [Google Scholar]
- Shi, YZ; Feng, QH; Ma, LF; Han, WY; Ruan, JY; Wang, F. Simultaneous Analysis of La, Ce, Pr, Sm and Nd in Tea with ICP-OES. Food Sci 2008, 29, 310–313. [Google Scholar]
- Fang, F; Wu, CZ; Hong, W; Fang, HL; Song, P. Study on the relationship between rhizospheric or rhizospheric soil enzyme and microbe in different plants. Subtrop. Agric. Res 2007, 3, 209–215. [Google Scholar]
- Ren, DQ; Suo, FM; Chen, SL. Research on the suitability evaluation of producing area of Dao-di Chinese material medica. Res. Inf. Tradit. Chin. Med 2005, 7, 4–9. [Google Scholar]
- Marschner, P; Crowley, DE; Yang, CH. Development of specific rhizosphere communities in relation to plant species; nutrition and soil type. Plant Soil 2004, 261, 199–208. [Google Scholar]
- Wang, H; Wang, G; Huang, YY; Cheng, J; Cheng, MM. The effect of pH changes on the activity of acid enzyme. Ecol. Environ 2008, 17, 2401–2406. [Google Scholar]
- He, B; Wen, YG; Yuan, X; Liang, HW; Liu, SR. Studies on soil physical and chemical properties and enzyme activities of different mangrove communitiies in Yingluo Bay of Guangxi. Scientia Silvae Sinica 2002, 38, 2l–26. [Google Scholar]
- Richard, PD. Defining soil quality for a sustainable environment. Soil Sci Soc Am 1994, 107–124. [Google Scholar]
- Yuan, XF; Xu, J; Chai, H; Lin, HR; Yang, YQ; Wo, XD; Shi, JY. Differences of rhizo-bacterial diversity and the content of peimine and peiminine of Fritillaria thunbergii among different habits. J. Med. Plants Res 2010, 4, 465–470. [Google Scholar]
- Fierer, N; Jackson, RB. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar]
- Liu, XF; Liu, XL; Chen, Q; Jiang, JY. The effect of mineral nutrietion and pH value on the growth of Fritillaria thunbergii. Zhejiang Prov. Agric. Sci 2008, 6, 699–701. [Google Scholar]
- Omidi, H; Tahmasebi, Z; Torabi, H; Miransari, M. Soil enzymatic activities and available P and Zn as affected by tillage practices; canola (Brassica napus L.) cultivars and planting dates. Eur. J. Soil Biol 2008, 44, 443–450. [Google Scholar]
- Bandick, AK; Dick, RP. Field management effects on soil enzyme activities. Soil Biol. Biochem 1999, 31, 1471–1479. [Google Scholar]
- Zimmerman, S; Frey, B. Soil respiration and microbial properties in an acid forest soil: Effects of wood ash. Soil Biol. Biochem 2002, 34, 1727–1737. [Google Scholar]
- Marschner, P; Grierson, PF; Rengel, Z. Microbial community composition and functioning in the rhizosphere of three Bandsia species in native woodland in western Australia. Appl. Soil Ecol 2005, 28, 191–201. [Google Scholar]
- Pitman, NCA; Jørgensen, PM. Estimating the size of the threatened world flora. Science 2002, 298, 989. [Google Scholar]
- Buchanan, BB; Gruissem, W; Jones, RL. Biochemistry and Molecular Biology of Plants; American Society of Plant Physiologists: Rockville, MA, USA, 2000. [Google Scholar]
- Arkhipova, TN; Veselov, SU; Meluntiev, AI. Ability of bactcrium Bacillis subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar]
- Smalla, K; Wieland, G; Buchner, A; Zock, A; Parzy, J; Kaiser, S; Roskot, N; Heuer, H; Berg, G. Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl. Environ. Microbiol 2001, 67, 4742–4751. [Google Scholar]
- Li, FD; Yu, ZN; He, SJ. Experimental Techniques in Agricultural Microbiology; Chinese Agricultural Press: Beijing, China, 1996; pp. 137–139. [Google Scholar]
- Zhou, LK; Zhang, ZM. Determination of soil enzyme activities. Chin. J. Soil Sci 1980, 5, 37–38. [Google Scholar]
- Yuan, XF; Shi, JY; Yang, YQ; Luan, J; Gao, JJ; Wang, YW. Establishment of element fingerprint and multielement analysis of Fritillaria thunbergii by inductively coupled plasma optical emission spectrometry. Biol. Trace Elem. Res 2010, 135, 304–313. [Google Scholar]
- Muyzer, G; Ellen, CW; Andre, GU. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction genes coding for 16S rRNA. Appl. Environ. Microbiol 1993, 59, 695–700. [Google Scholar]
- Lin, HR; Chen, XC; Hu, SP; Shen, CF; Chen, GC; Shi, JY; Chen, YX. Lead availability and soil microbial community composition in rice rhizosphere affected by thiosulfate addition. Appl. Soil Ecol 2010, 45, 232–237. [Google Scholar]




| Element | Rhizosphere Soil in Nantong | Bulk Soil in Nantong | Rhizosphere Soil in Panan | Bulk Soil in Panan | Rhizosphere Soil in Ningbo | Bulk Soil in Ningbo |
|---|---|---|---|---|---|---|
| Al | 5144 ± 402 | 3133 ± 376 | 8039 ± 366 | 3843 ± 249 | 9079 ± 427 | 5966 ± 310 |
| B | 3638 ± 262 | 2145 ± 285 | 1379 ± 404 | 570 ± 66 | 1215 ± 283 | 494 ± 76 |
| Ca | 2400 ± 166 | 2243 ± 225 | 1560 ± 71 | 994 ± 121 | 1763 ± 165 | 1031 ± 65 |
| Fe | 5041 ± 186 | 4856 ± 53 | 3637 ± 374 | 4734 ± 172 | 4705 ± 128 | 4349 ± 248 |
| K | 9261 ± 310 | 10,535 ± 458 | 13,393 ± 688 | 10,285 ± 321 | 11,450 ± 72 | 7139 ± 360 |
| Na | 3385 ± 348 | 4769 ± 169 | 5342 ± 470 | 4293 ± 252 | 5619 ± 188 | 3607 ± 323 |
| Mg | 309 ± 19 | 469 ± 16 | 75 ± 7 | 46 ± 11 | 168 ± 21 | 179 ± 15 |
| Mn | 258 ± 18 | 229 ± 5 | 167 ± 4 | 227 ± 30 | 199 ± 16 | 199 ± 17 |
| P | 546 ± 24 | 513 ± 13 | 369 ± 21 | 207 ± 11 | 915 ± 33 | 898 ± 24 |
| S | 162 ± 23 | 132 ± 7 | 91 ± 4 | 72 ± 4 | 183 ± 8 | 164 ± 2 |
| Co | 8.8 ± 0.5 | 10.2 ± 0.5 | 6.2 ± 0.6 | 7.3 ± 0.3 | 8.0 ± 0.7 | 9.2 ± 0.3 |
| Zn | 59.6 ± 7.9 | 71.1 ± 12.8 | 44.7 ± 10.5 | 38.4 ± 7.2 | 61.9 ± 4.9 | 55.1 ± 6.9 |
| Cu | 29.8 ± 2.5 | 36.4 ± 0.1 | 8.6 ± 0.2 | 3.9 ± 0.2 | 40.0 ± 1.3 | 17.6 ± 1.5 |
| Cd | 1.1 ± 0.1 | 0.5 ± 0.1 | 1.1 ± 0.0 | 0.7 ± 0.1 | 1.6 ± 0.1 | 0.8 ± 0.1 |
| Cr | 58.6 ± 6.1 | 57.7 ± 0.3 | 20.6 ± 0.9 | 22.1 ± 0.9 | 45.9 ± 3.2 | 34.4 ± 2.6 |
| Pb | 30.4 ± 2.8 | 26.8 ± 0.4 | 27.8 ± 2.1 | 23.8 ± 1.7 | 41.5 ± 3.9 | 30.9 ± 1.7 |
| Ca | Fe | K | Mg | Mn | Na | P | S | B | Co | Zn | Cu | Cd | Cr | Pb | OM | pH | Phosphatase | Urease | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | −0.26 | −0.49* | 0.36 | −0.60** | −0.56** | 0.41* | 0.47* | 0.27 | −0.22 | −0.60** | −0.19 | −0.06 | 0.85** | −0.36 | 0.68** | 0.53** | −0.73** | 0.93** | 0.24 |
| Ca | 1.00 | 0.47* | 0.09 | 0.77** | 0.51* | −0.14 | 0.00 | 0.42* | 0.86** | 0.46* | 0.47* | 0.72** | 0.00 | 0.83** | 0.13 | −0.24 | 0.78** | −0.47* | 0.63** |
| Fe | 1.00 | −0.44* | 0.55** | 0.84** | −0.43* | 0.07 | 0.37 | 0.55** | 0.59** | 0.51* | 0.57** | −0.15 | 0.75** | 0.05 | −0.23 | 0.63** | −0.62** | 0.33 | |
| K | 1.00 | −0.20 | −0.40 | 0.80** | −0.39 | −0.44* | −0.08 | −0.49* | −0.17 | −0.07 | 0.20 | −0.28 | 0.01 | −0.15 | −0.34 | 0.41* | −0.28 | ||
| Mg | 1.00 | 0.49* | −0.15 | 0.11 | 0.35 | 0.58** | 0.86** | 0.73** | 0.77** | −0.48* | 0.87** | −0.09 | −0.14 | 0.90** | −0.67** | 0.53** | |||
| Mn | 1.00 | −0.59** | −0.15 | 0.25 | 0.69** | 0.49* | 0.30 | 0.36 | −0.20 | 0.69** | −0.15 | −0.34 | 0.70** | −0.74** | 0.23 | ||||
| Na | 1.00 | 0.02 | −0.24 | −0.42* | −0.26 | 0.06 | 0.13 | 0.11 | −0.26 | 0.18 | 0.05 | −0.45* | 0.57** | −0.16 | |||||
| P | 1.00 | 0.84** | −0.11 | 0.31 | 0.36 | 0.53** | 0.42* | 0.29 | 0.74** | 0.69** | −0.12 | 0.44* | 0.73** | ||||||
| S | 1.00 | 0.35 | 0.43* | 0.42* | 0.70** | 0.43* | 0.62** | 0.70** | 0.45* | 0.25 | 0.11 | 0.89** | |||||||
| B | 1.00 | 0.33 | 0.43* | 0.50* | 0.07 | 0.77** | 0.01 | −0.28 | 0.75** | −0.54** | 0.56** | ||||||||
| Co | 1.00 | 0.70** | 0.67** | −0.54** | 0.76** | −0.06 | 0.03 | 0.75** | −0.61** | 0.47* | |||||||||
| Zn | 1.00 | 0.78** | −0.20 | 0.74** | 0.18 | 0.17 | 0.57** | −0.29 | 0.60** | ||||||||||
| Cu | 1.00 | 0.08 | 0.88** | 0.47* | 0.13 | 0.55** | −0.17 | 0.81** | |||||||||||
| Cd | 1.00 | −0.13 | 0.74** | 0.40 | −0.51* | 0.70** | 0.37 | ||||||||||||
| Cr | 1.00 | 0.17 | −0.07 | 0.84** | −0.55** | 0.74** | |||||||||||||
| Pb | 1.00 | 0.54** | −0.31 | 0.62** | 0.63** | ||||||||||||||
| OM | 1.00 | −0.33 | 0.54** | 0.37 | |||||||||||||||
| pH | 1.00 | −0.88** | 0.41* | ||||||||||||||||
| Phosphatase | 1.00 | 0.03 |
| Band | The Closest Sequences (GenBank Accession Number) | Length (bp) | NB | NB′ | PA | PA′ | NT | NT′ |
|---|---|---|---|---|---|---|---|---|
| T1 | Uncultured actinobacterium clone (EU715956) | 170 | 0 | 0 | 1 | 0 | 1 | 1 |
| T2 | Uncultured Sphingomonadaceae bacterium clone (GQ338772) | 169 | 0 | 0 | 1 | 1 | 1 | 0 |
| T3 | Uncultured Acidobacteria bacterium clone (EF663281) | 193 | 0 | 0 | 1 | 1 | 1 | 1 |
| T4 | Pseudomonas reactans strain PSR2 (GQ354529) | 194 | 0 | 0 | 1 | 1 | 1 | 0 |
| T5 | Acinetobacter sp. 71A1 (GQ178053) | 195 | 0 | 1 | 1 | 1 | 1 | 1 |
| T6 | Uncultured Sphingobacteriales bacterium (FJ536881) | 189 | 1 | 0 | 1 | 1 | 1 | 1 |
| T7 | Uncultured Acinetobacter sp. clone GI8-sp-H16 (GQ129954) | 195 | 1 | 1 | 1 | 1 | 1 | 1 |
| T8 | Flavobacterium columnare strain QJH-2 (EU395803) | 1 | 1 | 1 | 1 | 0 | 1 | |
| T9 | Acinetobacter sp. 423D (GQ178046) | 189 | 1 | 1 | 1 | 1 | 0 | 1 |
| T10 | Uncultured bacterium clone nbw691h12c1 (GQ116358) | 195 | 0 | 1 | 0 | 0 | 0 | 0 |
| T11 | Uncultured soil bacterium C0165 (AF128666) | 189 | 1 | 1 | 1 | 1 | 0 | 0 |
| T12 | Uncultured Acinetobacter sp. clone JEL30 (DQ130041) | 194 | 0 | 0 | 1 | 0 | 0 | 0 |
| T13 | Uncultured Bacteroidetes bacterium (GQ469472) | 195 | 1 | 1 | 1 | 1 | 1 | 1 |
| T14 | Rhodanobacter sp. IMER-B2-16 (FJ772029) | 189 | 0 | 1 | 0 | 0 | 0 | 0 |
| T15 | Uncultured bacterium clone S5-92 (EU680352) | 194 | 0 | 0 | 1 | 1 | 0 | 0 |
| T16 | Uncultured Rhizobiales bacterium clone P1s-1 (GQ287595) | 169 | 0 | 0 | 1 | 0 | 0 | 0 |
| T17 | Sphingomonas sp. DX-T3-03 (GQ895737) | 169 | 0 | 0 | 1 | 1 | 1 | 1 |
| T18 | Uncultured soil bacterium clone 20_5KE05 (GQ918621) | 169 | 1 | 1 | 1 | 1 | 1 | 1 |
| T19 | Uncultured bacterium clone CafTC09 (GQ401700) | 194 | 0 | 0 | 1 | 0 | 0 | 0 |
| T20 | Uncultured ammonia-oxidizing bacterium isolate (GQ281017) | 194 | 0 | 0 | 0 | 1 | 1 | 1 |
| T21 | Uncultured proteobacterium clone (DQ223199) | 194 | 1 | 0 | 0 | 0 | 1 | 0 |
| T22 | Aquicella lusitana strain SGT-39 (NR_025763) | 193 | 0 | 0 | 1 | 1 | 0 | 0 |
| T23 | Uncultured Firmicutes bacterium clone (EU299094) | 193 | 0 | 0 | 1 | 1 | 1 | 1 |
| T24 | Micrococcus sp. OS5 clone F12 (GU003860) | 193 | 1 | 1 | 1 | 1 | 1 | 0 |
| T25 | Uncultured soil bacterium clone 21_77KB12 (GQ918955) | 174 | 0 | 0 | 1 | 1 | 1 | 1 |
| T26 | Uncultured actinobacterium clone B10-05C (FJ543076) | 170 | 0 | 0 | 1 | 1 | 1 | 0 |
| T27 | Uncultured bacterium clone C007 (GQ495796) | 169 | 0 | 0 | 0 | 0 | 1 | 0 |
| T28 | Uncultured bacterium clone MACA-CC01 (GQ500694) | 170 | 1 | 0 | 1 | 1 | 0 | 0 |
| T29 | Uncultured Acidobacteria bacterium clone (GQ288459) | 194 | 1 | 1 | 1 | 1 | 0 | 0 |
| T30 | Uncultured soil bacterium clone 20_5KB11 (GQ918592) | 169 | 0 | 1 | 1 | 1 | 0 | 0 |
| T31 | Uncultured actinobacterium clone H140 (GQ504251) | 169 | 0 | 0 | 1 | 1 | 0 | 0 |
| T32 | Uncultured bacterium clone D10H_H03 (GQ376837) | 174 | 1 | 1 | 1 | 1 | 1 | 1 |
| T33 | Uncultured Caulobacterales bacterium clone (EU665092) | 169 | 0 | 0 | 0 | 0 | 1 | 0 |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shi, J.-Y.; Yuan, X.-F.; Lin, H.-R.; Yang, Y.-Q.; Li, Z.-Y. Differences in Soil Properties and Bacterial Communities between the Rhizosphere and Bulk Soil and among Different Production Areas of the Medicinal Plant Fritillaria thunbergii. Int. J. Mol. Sci. 2011, 12, 3770-3785. https://doi.org/10.3390/ijms12063770
Shi J-Y, Yuan X-F, Lin H-R, Yang Y-Q, Li Z-Y. Differences in Soil Properties and Bacterial Communities between the Rhizosphere and Bulk Soil and among Different Production Areas of the Medicinal Plant Fritillaria thunbergii. International Journal of Molecular Sciences. 2011; 12(6):3770-3785. https://doi.org/10.3390/ijms12063770
Chicago/Turabian StyleShi, Ji-Yan, Xiao-Feng Yuan, Hui-Rong Lin, Yuan-Qiang Yang, and Zong-Yuan Li. 2011. "Differences in Soil Properties and Bacterial Communities between the Rhizosphere and Bulk Soil and among Different Production Areas of the Medicinal Plant Fritillaria thunbergii" International Journal of Molecular Sciences 12, no. 6: 3770-3785. https://doi.org/10.3390/ijms12063770




